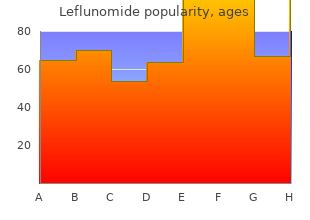
Air Force Institute of Technology. M. Yokian, MD: "Buy Leflunomide online in USA - Best Leflunomide OTC".
This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed purchase 10 mg leflunomide with amex medicine of the people, the full report) may be included in professional journals 131 provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising discount leflunomide 10 mg medicine 223. Applications for commercial reproduction should be addressed to: NIHR Journals Library generic leflunomide 10 mg with amex 4 medications, National Institute for Health Research buy 20mg leflunomide otc medicine overdose, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK. APPENDIX 4 • • • • • • 132 NIHR Journals Library www. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals 133 provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals 135 provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK. APPENDIX 4 • • • • • • • • • • • • • 136 NIHR Journals Library www. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals 137 provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK. APPENDIX 4 • • • • • • • • • 138 NIHR Journals Library www. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals 139 provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals 141 provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK. APPENDIX 5 • • o • o o • • o • • • • 142 NIHR Journals Library www. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals 143 provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals 145 provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK. APPENDIX 5 • • o o o o • o o • • • • • • o • o • 146 NIHR Journals Library www. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals 147 provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK. Stretching (including positional constraint-induced movement therapy) Eye-gaze skills stretching, splints) l Sensory/sensory integration l Adaptive/problem-solving skills Language development Endurance training (specific approaches mentioned: l Occupational performance coaching; Narrative/storytelling skills Cardiovascular fitness training Cognitive Orientation to daily Occupational Performance Reciprocal communication (e. Specific techniques: l Self-care/life skills baby-signing; intensive interaction) Constraint-induced movement Adjusting/changing a task to support a Aided Language Simulation therapy child to manage it independently Articulation therapy Bimanual training Providing equipment to enable child to engage in activities Breath support skills Proprioceptive neuromuscular facilitation l Seating Facial oral tract therapy l Postural management Hip and spine surveillance l Mobility (including powered) Dysphagia (swallowing, saliva control) l Small items (e. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals 149 provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK. APPENDIX 6 Physiotherapy Occupational therapy Speech and language therapy Hydrotherapy Changing the environment to support Augmentive and alternative engagement in activities or address communication systems Functional electrical stimulation care needs Feeding/drinking equipment Botulinum (botox) l Housing adaptations l Hoists Sports (e. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health Published by the NIHR Journals Library . He also reports a grant from the NIHR Public Health Research programme during the conduct of the study. Colin Green served as a member of the funding panel for the NIHR Programme Grants for Applied Research programme from 2009 to 2013. Colin Green (2007–13) and Siobhan Creanor (2013–present) served as members of the NIHR Research Funding Committee for the South West Region of the Research for Patient Benefit Programme. Intervention costs for this study were paid for by the Peninsula College of Medicine and Dentistry. Stuart Logan (NF-SI-0515–10062) and Rod Taylor (NF-SI-0514–10155) are NIHR senior investigators. This study was undertaken in collaboration with the Peninsula Clinical Trials Unit (CTU), a UK Clinical Research Collaboration-registered CTU in receipt of NIHR CTU support funding. None of the funders had any involvement in the Trial Steering Committee, data analysis, data interpretation, data collection or writing of the report. Cluster randomised controlled trial and economic and process evaluation to determine the effectiveness and cost-effectiveness of a novel intervention [Healthy Lifestyles Programme (HeLP)] to prevent obesity in school children. Public Health Research ISSN 2050-4381 (Print) ISSN 2050-439X (Online) This journal is a member of and subscribes to the principles of the Committee on Publication Ethics (COPE) (www. Print-on-demand copies can be purchased from the report pages of the NIHR Journals Library website: www. PHR programme The Public Health Research (PHR) programme, part of the National Institute for Health Research (NIHR), evaluates public health interventions, providing new knowledge on the benefits, costs, acceptability and wider impacts of non-NHS interventions intended to improve the health of the public and reduce inequalities in health. The scope of the programme is multi-disciplinary and broad, covering a range of interventions that improve public health. The Public Health Research programme also complements the NIHR Health Technology Assessment programme which has a growing portfolio evaluating NHS public health interventions. For more information about the PHR programme please visit the website: http://www. The final report began editorial review in November 2016 and was accepted for publication in February 2017. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. However, they do not accept liability for damages or losses arising from material published in this report. This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the PHR programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the PHR programme or the Department of Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK. Public Health Research Editor-in-Chief Professor Martin White Director of Research and Programme Leader, UKCRC Centre for Diet and Activity Research (CEDAR), MRC Epidemiology Unit, Institute of Metabolic Science, School of Clinical Medicine, University of Cambridge; Visiting Professor, Newcastle University; and Director, NIHR Public Health Research Programme NIHR Journals Library Editor-in-Chief Professor Tom Walley Director, NIHR Evaluation, Trials and Studies and Director of the EME Programme, UK NIHR Journals Library Editors Professor Ken Stein Chair of HTA and EME Editorial Board and Professor of Public Health, University of Exeter Medical School, UK Professor Andrée Le May Chair of NIHR Journals Library Editorial Group (HS&DR, PGfAR, PHR journals) Dr Martin Ashton-Key Consultant in Public Health Medicine/Consultant Advisor, NETSCC, UK Professor Matthias Beck Professor of Management, Cork University Business School, Department of Management and Marketing, University College Cork, Ireland Dr Tessa Crilly Director, Crystal Blue Consulting Ltd, UK Dr Eugenia Cronin Senior Scientific Advisor, Wessex Institute, UK Dr Peter Davidson Director of the NIHR Dissemination Centre, University of Southampton, UK Ms Tara Lamont Scientific Advisor, NETSCC, UK Dr Catriona McDaid Senior Research Fellow, York Trials Unit, Department of Health Sciences, University of York, UK Professor William McGuire Professor of Child Health, Hull York Medical School, University of York, UK Professor Geoffrey Meads Professor of Wellbeing Research, University of Winchester, UK Professor John Norrie Chair in Medical Statistics, University of Edinburgh, UK Professor John Powell Consultant Clinical Adviser, National Institute for Health and Care Excellence (NICE), UK Professor James Raftery Professor of Health Technology Assessment, Wessex Institute, Faculty of Medicine, University of Southampton, UK Dr Rob Riemsma Reviews Manager, Kleijnen Systematic Reviews Ltd, UK Professor Helen Roberts Professor of Child Health Research, UCL Institute of Child Health, UK Professor Jonathan Ross Professor of Sexual Health and HIV, University Hospital Birmingham, UK Professor Helen Snooks Professor of Health Services Research, Institute of Life Science, College of Medicine, Swansea University, UK Professor Jim Thornton Professor of Obstetrics and Gynaecology, Faculty of Medicine and Health Sciences, University of Nottingham, UK Professor Martin Underwood Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick, UK Please visit the website for a list of members of the NIHR Journals Library Board: www.
Author Books which are not freely available on the internet are like cars without wheels buy generic leflunomide 10 mg on-line medicine 2015 song. Doctor As you can see order leflunomide 20 mg free shipping symptoms bone cancer, publishers who take their task seriously and want to be more than just a figurehead have plenty to do order 10 mg leflunomide amex medicine daughter lyrics. The world needs one hundred doctors Student If you have heard of Flying Publisher projects and have basic skills in the management of HTML sites buy leflunomide 10 mg mastercard treatment 6th february, go ahead and offer your services. By collaborating on a textbook project you will learn things from which you will benefit for the rest of your lives. Bystander The printing costs for books may be lower than you thought. If a printing machine is set up and the first 1000 copies have been printed, it costs €3. In fact – as we will see later on – a publisher can only pay his authors an appropriate fee of 25 Euro per page, for example, if he sells more than 1000 copies. Getting the train on the track Book format – Against the light – References – Journalistic handbooks – Styles – Key combinations – Letters to the authors – Kick-off – List of contributors – Bank details The editorial team is complete – lectorship, secretariat, mentor, proofreaders – and the authors of your choice have agreed. The authors need four more things before kick-off: A document into which they can insert their text, A set of instructions for the compilation of the references, Assistance with writing (style and technique), and The starting signal together with the deadline. Document for the texts The authors must not be allowed to write at random, but must write their texts into a template supplied by you. An exemplary template can be found on the internet under www. There, you will find examples of tables, diagrams, frames and reference lists. Before you send the template to your authors, you must define the book format, because the maximum width of tables and diagrams depends on this format. Over a glass of red wine in your library, you should decide how high and how wide your book needs to be. HIV Medicine 2005 has the dimensions 15 cm x 24 cm, Free Medical Information 13 cm x 21 cm and the pocket edition of HIV Medicine 11 cm x 18 cm. For a book such as Free Medical Information, you would find 2. In the menu “Apply to” (bottom right in the dialogue window) select “Whole document”. Then define the margins according to the size of book required (see Table 3. Put the printouts on top of the book that felt so good in your hand and hold it up against the light. In half an hour, you will have decided what size your book will be. Before you send your authors the template for the text, you must prepare two more things. Firstly, instructions for the compilation of the references and secondly, instructions on how to write well. This sounds like a very simple statement, but is in fact difficult. It is never too soon to commit the authors to uniform procedures. We have wasted many hours correcting the references. The New England Journal of Medicine, for example, uses the format surname, initial of first name, et al. For example: Rockstroh JK, Mudar M, Lichterfeld M, et al. Pilot study of interferon alpha high- dose induction therapy in combination with ribavirin for chronic hepatitis C in HIV-co-infected patients. There are more details in these three lines than most authors can cope with without help: There is no full stop after the initials of first names; several initials are written together. If there are more than 6 authors, the first 3 are named, then comes a comma, followed by “et al” and finished with a full stop. After the title is a full stop (rarely a question or exclamation mark). N Engl J Med for New England Journal of Medicine, BMJ for British Medical Journal. After the journal comes the year, separated only by a space. Only the end digits of the last page number, which are necessary for clear identification. Thus, 2423-2429 becomes 2423-9, 134-141 becomes 134-41, 1891-1901 becomes 1891-901. Do not be afraid to explain the format of the references in equally great detail. You will save both yourself and your authors a lot of work in the proofreading phase. Assistance with writing Doctors are grateful for assistance with writing, despite A levels, a medical degree and post-doctoral lecture qualification. Style Some suggestions, very general and valid for every subject: The important things come first, the unimportant ones later. Procedure: first, collect material, then sort the facts, and finally structure them. Do not write until it is clear to you what you want to express. The medical faculty is not a place which insists on stylistic skill. Technique The number of text elements which you require for your textbook is limited. Apart from the normal text (that is the text you are reading at the moment), we only used the following text styles in HIV Medicine 2005, for example: Normal Heading 1 Heading 2 Heading 3 Heading 4 Table Table heading References 40 Assistance with writing Headings 1 to 4 are the chapter headings according to the hierarchical structure. The most important rule is: you must never – and this applies to your authors as well – change typeface or type size via the pop-down menus shown in Fig. Instead, you should change them via the menu styles (Fig. Among other things, a style contains information about type size, typeface, and also line spacing between your text and the previous and subsequent text section. If you want to change the style of a paragraph, you just have to position the cursor somewhere in the paragraph. If you want to allocate a style to several paragraphs, you must mark the paragraphs first. The advantage of styles: later, you can alter your entire text in a matter of minutes, simply by changing type size and spacing for the individual styles.
Further leflunomide 20mg without prescription medications quizlet, the laboratory of nergic tone and through that enhancement achieve some buy 20 mg leflunomide with amex treatment example, Nestler showed that accumulation of chronic FRAs during most 10 mg leflunomide overnight delivery symptoms nerve damage, or all of their reinforcing effects purchase leflunomide 10 mg visa medicine park cabins. Moreover, through chronic morphine treatment, related to the transient early a variety of studies, primarily conducted in animals using gene protein products Fos and Jun, which, in turn, join to either surgical lesions or specifically directed neurotoxins, form a major gene transcription factor, activator protein 1, and also other specific chemicals to enhance or decrease may play a role in the effects of morphine and also of stimu- dopaminergic function, along with ultimately microdialysis lants (18). Using an effective construct involv- forcing effects of most or all drugs of abuse. This gene construct allows overproduction of FosB tion of those neurons (62). Thus, by inhibiting these inhibi- by the gene insertion, the overexpression of which can be tory neurons, which normally put a brake on the dopami- prevented by administration of a tetracycline congener, but nergic neurons in the ventral tegmental area, the result is it be started again by stopping treatment with the tetracy- activation of the dopaminergic neurons, with enhanced re- cline congener. The overexpression can be both brain region lease of dopamine in the nucleus accumbens, as well as in specific and time specific (12). To date, enhancement of the amygdala and probably in all other regions of the meso- FosB in the striatum has been shown to alter the behav- limbic-mesocortical dopaminergic fields (62). Using a different transgenic Although many investigators have attributed the rein- approach, a viral vector may be used to deliver a desired forcing effects of all drugs of abuse, including heroin and gene to a specific brain region to yield overexpression. There is an increasing consensus that the reinforcing ef- Studies have been conducted in animals with deletion fects of drugs of abuse, along with possibly physical depen- of the dopamine transporter gene, which many researchers dence, are not directly related to tolerance, and they also had hypothesized would eliminate cocaine self-administra- may not be directly related to any changes in receptor den- tion because of the very high constant levels of dopamine sity, number, desensitization, internalization, G-protein un- and the lack of further effects by superimposed cocaine (63). These findings are further supported by the report transporter knockout mice were found unequivocally to of Bohn, Lefkowitz, Caron, and colleagues that, in studies self-administer cocaine, although the acquisition of that be- in -arrestin knockout mice, one sees enhancement and havior was slower than in the wild-type mice (64). Thus, persistence of the antinociceptive effects of morphine (60). In that same animal model, the dopamine tion of opiates and no rewarding effects of opiates (reviewed reuptake transporter knockout mice, it has been found, in ref. Hemby and Smith in dopamine D1 mRNA levels, was found at the end of the and their colleagues also found a synergistic elevation of 6 days of morphine exposure (70). The mRNA levels for extracellular dopamine when cocaine was added to heroin both dopamine D1 and D2 receptors was reduced after 1 in self-administration studies (66). These findings may ex- day of withdrawal, and both returned toward normal by plain, in part, the common co-dependency in humans of the third day after drug withdrawal. These findings may be both heroin and cocaine addictions. However, curiously in this neurons completely in discrete brain regions by use of a study, but not in other studies, reductions of mRNA levels neurotoxin, 6-hydroxydopamine, self-administration of for dynorphin and enkephalin genes were found during morphine proceeded normally as in unlesioned animals. In contrast, enhanced dynorphin However, in such animals, cocaine self-administration was mRNA levels have been found at least after acute single and eliminated. Further studies will be needed to determine the time instance, in one study using the technique of in vivo fast course of dynorphin mRNA level changes during morphine cyclic voltammetry, it was found that heroin caused a dose- exposure. Trujillo, Akil, and their colleagues showed that dependent increase in dopamine in the nucleus accumbens chronic injection or infusion of morphine caused increases during heroin self-administration, and co-administration of in levels of dynorphin peptides in the dorsal striatum (cau- a -agonist (U-50,488 H) with the heroin, or alternatively, date putamen) but not in the ventral striatum (nucleus ac- intracerebroventricular administration of dynorphin A, sig- cumbens) (73). Moreover, installation of the -synthetic compound or only a few (approximately 20%) nucleus accumbens neu- natural ligand dynorphin A alone decreased basal dopamine rons seem to exhibit an inhibitory response after heroin self- release, as had also been shown by Claye and others (68). Thus, the multiple -agonist morphine activates the mesolimbic-mesocortical changes in signal transduction observed and discussed ear- dopaminergic pathway and that -opioid-receptor activa- lier, including the effects of chronic morphine administra- 35 tion offsets, or counterregulates, that activation (67). In related studies, these investigators result from a direct opiate effect or an indirect effect by found, as have numerous others, that cocaine caused a strik- alteration of the dopaminergic system (40,42). Similar find- ing increase in extracellular dopamine concentrations in the ings were made by the group of Sim-Selley, Selley, Childers, nucleus accumbens, and, moreover, the combination of co- and colleagues after chronic heroin self-administration, with caine and heroin caused a synergistic elevation (66). Their the greatest decrease in -opioid-receptor–stimulated 35 finding that heroin alone failed to cause an increase in dopa- [ S]GTP S binding in the brainstem and the lowest altera- mine in the nucleus accumbens complemented several ear- tions in binding in the striatum and cortex (42). Because lier findings that heroin self-administration is not attenu- the changes of dopamine D1-receptor activation would act ated by administration of dopamine antagonists, as well as in one direction and dopamine D2-receptor activation even earlier studies showing that integrity of dopamine would act in the opposite direction on adenylyl cyclase activ- pathways in the nucleus accumbens is not essential for her- ity, the effects on these receptors could also influence the oin self-administration. These findings document further effects of -opioid-receptor activation, and the changes that the early hypothesis of the Kreek laboratory, and many oth- have been observed may result exclusively from the opioid ers, that the reinforcing properties of heroin are mediated effects acting at the -opioid receptors or also secondary primarily by dopamine-independent mechanisms and prob- indirect effects on dopamine receptors. This hypothesis has These and other findings suggest that opiates may act 1496 Neuropsychopharmacology: The Fifth Generation of Progress directly to alter dopaminergic systems both in the ventrome- that is, the neuroplasticity after chronic opiate administra- dial striatum, that is, the core and shell of the nucleus ac- tion that results in impairment of normal neural integrity. Clearly, there are abundant - regulatory events may alter neural growth, development, opioid receptors as well as -opioid receptors in those re- and synapse formation, signal transduction, and overall sys- gions (26,75–77). Work from the Kreek laboratory showed tem integrity (24,79). There have been no similar expression, as well as more direct effects of enhanced tran- findings with respect to increasing -opioid-receptor den- scription factors on dynorphin gene expression, may be sity after chronic opioid administration, however. It is not again important counterregulatory events, which also repre- really known to what extent reinforcement or reward result- sent examples of profound neuroplasticity of the brain. Such ing from heroin and morphine occurs because of activation findings have also been made during binge pattern cocaine directly in these areas, especially the nucleus accumbens and administration (80,81). Enhanced dynorphin peptides, in possibly also the amygdala, as contrasted to indirect effects turn, acting at -opioid receptors, may reduce dopaminergic on the ventral tegmental area. The effects on dopamine tone in many brain regions, including those involved in in each of these different locations and also the different reward and also locomotor activity, and they may also atten- mechanisms involved have not yet been fully elucidated uate opioid withdrawal in dependent animals or humans using a model of chronic, high-dose, intermittent but evenly (6,8,9–11,16). Again, these events must be considered to spaced opiate administration, mimicking the human pattern be a direct result of neuroplasticity and are counterregula- of heroin or morphine abuse and addiction, and also after tory, the attempt to attenuate, modulate, or even brake the withdrawal, as well as during reexposure after such opiate events caused by the rapid changes in dopaminergic tone administration. During chronic binge pattern cocaine ad- brought about especially by stimulants such as cocaine, but ministration, a pattern mimicking the human condition, also to a lesser extent also by opiates. Noble edly primarily the result of the effects of chronic opioid and Cox clearly defined a role of the dopaminergic system in administration. However, because there are also significant opioid-receptor desensitization in these brain regions during changes in dopaminergic tone with enhanced signaling chronic morphine administration (39). This is a rate-limiting enzyme in the biosyn- tion of the dopaminergic neurons, as stated earlier. They also found a reduction in mean D5-type dopaminergic receptors enhance adenylyl cyclase size of the ventral tegmental area dopaminergic neurons and activity, an effect similar to that occurring in the locus ceru- decreased axonal transport to the nucleus accumbens (24, leus after chronic, but not acute, morphine administration, 79). However, there were no changes in numbers of dopa- in most strains of rodents studied, and also in the nucleus minergic neurons and no changes in the size of nondopami- accumbens in some strains of some species. Within ventral tegmental area, infusion activation of the dopaminergic D2 receptors causes a reduc- of brain-derived neurotrophic factor prevented this mor- tion in adenylyl cyclase activity, such as observed during phine-induced reduction in size of dopaminergic neurons acute morphine administration in all brain regions of strains (79). Their group also found that chronic morphine admin- and species of rodents studied, as well as in all cell systems istration resulted in an increase of other components related studied, and an effect that continues to pertain in some to signal transduction, including the extracellular signal reg- specific regions of the brain and other parts of the body ulated kinases (ERKs), which are effectors for brain-derived during chronic opioid administration. Thus, the observa- neurotrophic factor in the ventral tegmental area (24). Crain There have been only limited studies of the time course and Shen hypothesized that, although most -opioid recep- of all these dopaminergic responses during investigator-ad- tors are coupled with inhibitory Gi/oprotein, a small propor- ministered morphine or heroin on an intermittent basis, tion may be coupled at the stimulatory Gs protein, which mimicking the human pattern of heroin abuse, or during can be suppressed with small amounts of opioid antagonists. It would be assumed These findings of enhanced morphine analgesia are, in part, that possibly, as with cocaine, one sees a progressive diminu- very similar to the findings of Bohn, Caron, Lefkowitz, and tion of the responsivity, with a resultant lowering of basal colleagues, in mice with deletion of -arrestin (60). These level and stimulant-induced rise of absolute levels of dopa- researchers also showed that -arrestin is important in sev- mine (78). Numerous human studies suggest this may in- eral distinct functions, including events leading to the inter- deed happen. It has been repeatedly shown in heroin addicts nalization of an agonist bound -opioid receptor, which, that the short acting -opioid agonist heroin will cause a after the phosphorylation of the bound form, binds to - prompt increase in serum prolactin levels, resulting directly arrestin, along with binding by G-protein–receptor kinases from an abrupt lowering of dopamine levels in the tuberoin- (60). This event of -arrestin binding has been described fundibular dopaminergic systems (85). In humans, and to a as potentially part of the process that desensitizes, that is, greater extent than in rodents, prolactin release is essentially leads to G-protein uncoupling of the -opioid receptors, solely under tonic inhibition by dopaminergic tone in the as well as being actually involved in the internalization of tuberoinfundibular dopaminergic system.
Some participants expressed concern that they were unlikely to have the time and capacity to update the information following the conclusion of the project buy discount leflunomide 10 mg 7 medications that can cause incontinence. Nurse interview buy leflunomide 20mg fast delivery symptoms ms, participant 745 58 NIHR Journals Library www purchase 10 mg leflunomide mastercard medicine 031. Nurse interview 10 mg leflunomide amex symptoms lupus, participant 41 Participants also talked about copying and sharing the resource pack across the practice, and with colleagues who were not part of the research, as it was found to be of benefit for other staff to also be able to access: It was very easy. We were just saying that it would be good to copy it and let other staff in the practice use it too. Nurse interview, participant 178 Overall, there was a sense that the nurses found the resource pack very useful and had been active in signposting patients to various supports. This seemed to be accompanied by an approach of helping patients to access support for themselves and to address what their own priorities were, rather than focusing on fixing clinical issues: For patients to take the responsibility of looking after themselves with support from us and the better we can support them then hopefully the easier they will find it to take on the responsibility for their own health. Nurse interview, participant 745 Intended future use of the Patient Centred Assessment Method Participants were asked to reflect on their intentions around integrating PCAM-based consultations into their ongoing practice, beyond the course of the research project. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals 59 provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK. STUDY D: NURSE AND PATIENT PERCEPTIONS OF USING THE PATIENT CENTRED ASSESSMENT METHOD No participant in their feedback stated that they were opposed to using the PCAM in the future or made any comments indicating that they would be avoiding using the PCAM. Long-term adoption of the PCAM appears likely for some of the nurse participants in this research, beyond the research project itself. Conclusion The PCAM implementation did not have a negative or obstructive impact on the consultation. There was some indication that a small number of patients may have been aware of more discussion about their lives and their broader concerns. The PCAM training was acceptable, but required a multifaceted approach to training that integrated key information/knowledge, role playing and opportunities to apply the training to real consultations. Future training delivery will require the incorporation of these different aspects, and in a way that is flexible with nurse availability and workload. Overall, it appears that the PCAM was fairly easily integrated into consultation, although some participants reflected that the process of integration took some time and support, which will need to be taken into account in future training and support. The nurse participants perceived this to be beneficial for both the patient and the nurse, in relation to both the quality of the relationship and the quality of the care provided. Resource packs were seen as integral to using the PCAM, and practices engaged with these resources, often to the extent of taking ownership of their continuing development. However, for some practices, this could be seen as a future problem (how to keep these resources up to date) that could have an impact on their use of the PCAM. Long-term adoption of the PCAM was seen as feasible and possible by some nurses, which indicates overall potential for the acceptability and feasibility of the PCAM for use in primary care nurse-led consultations. The data used for the overall evaluation comprised: l contributions from study A focus groups with practices and patients on the acceptability and feasibility of the use of the PCAM, and any early reflections on barriers to using the PCAM in PN consultations l researcher field notes of meetings and discussions with staff l any comments to the research team or reported by practice staff from patients during implementation l data from study D, the final interviews with practice staff and patients l data from open-ended questions on staff and patient questionnaires collected as part of study B. The methods for studies A, B and D have been reported elsewhere. The collection of study field notes was seen as a fundamental part of the process evaluation and was ongoing throughout the study. Each study researcher kept their own logbook of visits and contacts with practices and any reported incidents/ problems, actions or comments associated with the study. The two researchers (EC and PA) had the most contact with practices, and each had more of a relationship with particular practices, thereby building rapport with practice staff and facilitating catch-up telephone or e-mail conversations between practice visits. However, this relationship was not exclusive to a single researcher and, therefore, all researchers, including Carina Hibberd, had some familiarity with all practices. The research team met regularly to discuss any incidents/problems, actions or comments from each site in order to compare notes and reflect on observations. This alerted each practice contact to possible similar problems or allowed reflection on possible solutions based on experiences within other practices. However, close contact was still required with CAU practices to ensure that data collection was still being achieved. This model was helpful in guiding the areas of knowledge needed for the process evaluation, but was also helpful in the analysis stage in pulling together knowledge from across the relevant data sets to articulate the context, implementation and mechanisms of impact within and across practices. Field note analysis The analysis of study A, B and D data sets has been described elsewhere in this report. The contributions of each of these data sets to the process analysis were initially discussed by several members of the research team (MM, CH, EC, PA, RP), and key relevant points for the implementation of both the PCAM and trial methods were agreed and summarised in accordance with the key components in the Moore et al. The field note data were extracted from researcher logbooks into summary tables for each practice, one table reflecting on the trial process (for all six practices) and one table reflecting on the PCAM implementation (for three practices). This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals 61 provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK. STUDY E: PROCESS EVALUATION any further discussion was needed across the team. The summary tables were organised around the Moore et al. For implementation of the PCAM, this included: l training and support l broader multidisciplinary team (MDT) involvement l the review/patient consultation l resources. For trial implementation this included: l training and support for data collection l broader practice involvement l patient conversations about the study. Within each of these broad areas, the researchers then grouped their field note data around topics of context, fidelity to study intentions, dose (in relation to trial training or intervention training), adaptations, reach (who did nurses include/exclude), unanticipated consequences and participant responses/interactions with the study. The research team then collectively reflected on these processes to determine key learning points for the implementation of the PCAM and the implementation of the trial. If time had allowed, we would have attempted to populate these tables, and followed this with a review of how best the broad areas and their topics consistently captured and described all the necessary detail, with a further iteration of the tables for final population/data extraction. Not all topics were populated across all practices, and some duplication of information occurred across categories, for example, some reflections on participant responses to/interactions with the study could also populate the topic of adaptations. This method of analysis of multiple researcher field note data was novel and could be refined for further studies. From feasibility to full-scale trial: using the ADePT decision aid to help identify protocol changes The true value of any feasibility study is to identify and/or address any threats to internal or external validity that may have an impact on a full-scale trial. However, very few feasibility studies assess this in any systematic way. We chose to reflect on our study and its process evaluation with reference to ADePT,51 to identify protocol modifications for a full-scale study. The ADePT algorithm seeks to encourage systematic identification and appraisal of problems and potential solutions, improve transparency of decision-making processes and reveal tensions that exist between choices that lead to a pragmatic versus explanatory trial. We identified the following areas as relevant to this feasibility trial: sample size, eligibility, recruitment, consent, randomisation, adherence/fidelity of intervention, acceptability of intervention, selection of appropriate outcomes, completion of outcomes, retention and logistics of multicentre sites and whether or not all components of the protocol worked. In evaluating the feasibility trial and its methods, several of the methodological issues identified by Shanyinde50 have been addressed elsewhere. Chapter 4 has addressed the quantitative findings in relation to sample, eligibility, recruitment, consent, randomisation and outcomes; Chapter 5 has addressed some aspects of adherence and fidelity to the PCAM intervention; and Chapters 3 and 6 have explored the acceptability of the PCAM intervention. The following analysis will focus on: l retention l study logistics in the multicentre sites l where any adaptations to protocol were identified l any unintended consequences l whether or not components of the protocol worked together.
Purchase leflunomide 20 mg on-line. SHINee (샤이니) 1 of 1 Lyrics (Han|Rom|Eng) Color Coded.