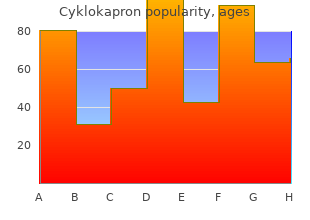
Cabrini College. W. Ismael, MD: "Buy online Cyklokapron - Effective online Cyklokapron".
The selection of the approach to be used has to be performed according to each case of therapeutic enzyme delivery mediated by enzymosomes trusted 500mg cyklokapron medications without a script. Acylation of Enzymes to Promote Hydrophobic Interaction with Liposomes The conjugation of a hydrophilic enzyme to acyl chains (Ac-enzyme) switches the affinity of the enzyme from hydrophilic to hydrophobic microenvironments (11 cyklokapron 500 mg free shipping medicine net,38) discount cyklokapron 500 mg without prescription treatment depression. The level of hydrophobicity of the Ac-enzyme is modulated by the number and/or the length of fatty chains linked to the enzyme surface cheap cyklokapron 500 mg treatment ulcerative colitis. The preservation of other properties of the modified enzyme is dependent on suitable strategies during con- jugation. An example is the case of Ac-l-asparaginase, which preserves 100% of the catalytic activity if the active site is blocked with the substrate during conjuga- tion (38,39). To maximize the load of such an Ac-enzyme into a liposome structure, 40 Cruz et al. Shown here is the localization both in the internal aqueous space (A) and in the lipid bilayer (B) of liposomes and the corresponding release (C and D). In case A, the enzyme is not available for substrate degradation when the liposome is intact; in case B, the enzyme is available even before liposome disruption. The Ac-enzyme can be partially inserted into the liposome bilayer or buried into the hydrophobic lipid matrix of the vesicles, which depends on the number and localization of the hydrophobic chains linked to the enzyme surface. The new procedure was developed to incorporate the bioconjugate Ac-l-asparaginase into liposomes (40). After the extrusion step used to reduce the size of the enzymosomes, any Ac-enzyme not incorporated is removed by gradient cen- trifugation. The Ac-enzyme–liposomal bilayer association depends on the overall electrostatic interactions between the enzyme- associated charges and on the hydrophobic interactions. The incorporation of Ac- enzymes into the bilayer of liposomes is efficiently evaluated by the ratio between the catalytic activity quantified in intact versus disrupted enzymosomes. No significant activity was found in intact liposomes loaded with the native enzyme [Fig. Chemical Link of Enzymes Directly to Liposome Surface As mentioned before, the other approach to build enzymosomes is by directly linking the hydrophilic enzyme to lipids of the liposome bilayer. The direct con- jugation of therapeutic enzymes to the outer surface of lipid vesicles remains a challenge, as few publications report the construction of liposomes with surface- attached enzymes. In contrast, many publications report the attachment of antibod- ies to the liposome surface, a concept widely used for the active targeting of lipo- somes (14,37). A suitable enzyme load, keeping the vesicle structural integrity and preserving the enzyme activity, was achieved (43). They combine the advantages of other carrier systems, especially regarding lipophilic drug incorporation and parenteral administration. They represent an alternative to polymeric particulate systems and are considered alternative carriers for pep- tides, proteins, and antigens. These colloidal systems are made from solid lipids (highly purified triglycerides, complex glyceride mixtures, or waxes) and stabilized by surfactant(s). There is no need for potentially toxic organic solvents for their production, which is impor- tant in protein formulation. Other procedures were described: solvent emulsification/evaporation method (50) or emulsification/diffusion technique (51), water/oil/water double- emulsion method, or high-speed stirring and/or ultrasonication technique. Super- critical fluid technology has recently been used to prepare lipid particles. Among these, loading onto preformed lipid nanoparticles by sorption procedures has also been introduced. In spite of lack of release mechanism knowledge and kinetic characterization, the prolonged in vitro release, and subsequent in vivo sustained effect of various proteins are described (46). Polymeric nanoparticles are obtained by different processes based on two main approaches: polymerization reactions and the use of preformed polymers (56,57). Nanospheres are defined as a polymeric matrix in which the drug is uni- formly dispersed and nanocapsules are described as a polymeric membrane that surrounds the drug in the matrix core (58). Their nanoparti- cles are easily obtained by an emulsion polymerization process developed by Cou- vreur (64). Owing to the structural complexity of enzymes, for their incorporation in nanoparticles, both the interaction of the enzyme with the compo- nents of the emulsion polymerization system and the effect of the process of poly- merization on the characteristics of the enzyme must be taken into account. Mild conditions are required, and each process must be optimized for each enzyme to maximize the enzyme load and minimize the loss of catalytic activity. The more obvious advantage of the emulsion polymerization is the absence of organic sol- vents. Conformational changes of the enzyme with consequent partial inactivation or strong modification of the kinetics are the main drawbacks. In brief, the monomer was added under stirring to the polymerization medium in which an amount of enzyme was added. In the double-emulsion method, enzymes in the aqueous solvent were emulsified with nonmiscible organic solution of the polymer to form a w/o emulsion. The organic solvent dichloromethane was mainly used and the homogenization step was carried out by using either high-speed homogenizers or sonicators. A homogenization step or intensive stirring is necessary to form a double emulsion of w/o/w. Then, the removal of organic solvent by heating and vacuum evaporation is done by either extracting organic solvent or adding a nonsolvent (i. The first process is designated as w/o/w, whereas the second is known as the phase-separation technique. In the spray-drying technique, parti- cle formation is achieved by atomizing the emulsion into a stream of hot air under vigorous solvent evaporation. Enzymes encapsulated into nanoparticles by w/o or w/o/w techniques are susceptible to denaturation, aggregation, oxidation, and cleavage, especially at the aqueous phase–solvent interface. Improved enzymatic activity has been achieved by the addition of stabilizers such as carrier proteins (e. The nanospheres obtained could continuously release the enzyme while preserving the enzymatic activity (74). These results were attributed to a favorable interaction of the enzyme with this specific copolymer (74,75). Transdermal drug delivery has been approved and has become widely accepted for the systemic administration of drugs. This noninvasive approach avoids the hepatic “first-pass” metabolism, maintains a steady drug concentration (extremely important both in the case of drugs with a short half-life and in the case of chronic therapy), allows the use of drugs with a low therapeutic index, and improves patient compliance. For charged and polar molecules or macro- molecules, skin delivery is difficult and has advanced substantially within the last few years. To facilitate the delivery of such entities, a number of strategies were developed.
The overall operational plan for phasing out d4T should be fully costed and should consider any additional investment in laboratory strengthening and capacity- building that may be required to support implementation buy cheap cyklokapron 500 mg medications not to take when pregnant. Because of programme constraints generic 500 mg cyklokapron visa symptoms 6 year molars, not all countries may be able to promptly switch everyone receiving d4T to new regimens buy generic cyklokapron 500mg on line medicine 5000 increase. Although new d4T orders should be discontinued cyklokapron 500 mg with mastercard treatment 1st degree av block, adequate and timely forecasting and procurement of the preferred alternative drug are critical to avoid stock-outs and treatment interruption. Countries are encouraged to ensure they are procuring these drugs at the best possible price. Options include reserving stocks for back-up situations for individuals who may require d4T in the absence of alternative choices. This section provides a broad outline of possible sequencing approaches to phasing in key recommendations, considering the available scientifc evidence, results from mathematical models (Box 10. It draws on views expressed in the Guidelines Development Group on Programmatic Issues and therefore does not constitute formal recommendations. National stakeholders are responsible for the process of revising and adapting the guidelines, and different approaches may be necessary and equally valid. This requires addressing any structural barriers that may prevent these populations from seeking and accessing care. In addition, as in concentrated epidemics, it important to identify and reach key populations and those with poor access to clinical and community-based services. These may include sex workers, people who inject drugs, men who have sex with 218 Consolidated guidelines on the use of antiretroviral drugs for treating and preventing hiv infection men, transgender people or other groups such as adolescent girls, migrants and other mobile populations, older women and certain high-risk occupational groups. Scaling up viral load monitoring will be important to adequately identify treatment failure and to avoid switching unnecessarily to second-line regimens. As people initiate treatment earlier and stay on it for longer, monitoring the quality of service delivery and strengthening service linkages to improve retention throughout the cascade of care are essential to optimize treatment outcomes and long-term programme performance. The key inputs required are the distribution of the adult population by risk group (such as stable couples, those with casual partners, female sex workers, male clients of sex workers, men who have sex with men, transgender people and people who inject drugs); sexual behaviour by risk group (numbers of partners per year, acts per partner and condom use) and needle sharing among people who inject drugs. Goals models already exist for about 25 countries, and other countries have compiled these data in the context of modes of transmission studies. OneHealth is a software tool designed to strengthen health system analysis and costing and to develop fnancing scenarios at the country level. It is specifcally designed to assess health investment needs in low- and middle-income countries and provides planners with a single framework for planning, costing, impact analysis, budgeting and fnancing of strategies for all major diseases and health system components. Several are available for download, with a description of their main purposes and programmatic focus (25). A fexible tool for costing investments in critical enablers (such as integrated treatment and rights literacy programmes, legal services, stigma and discrimination reduction programmes, training for health care workers and law enforcement) has also been developed and can be downloaded for free, along with a user guide (27,28). Such information is essential to detect and respond to bottlenecks or gaps in programme performance and to adequately characterize and respond to patient attrition. As programmes mature, monitoring individual- and population-level outcomes, including toxicity and adverse events, drug resistance, viral suppression, mortality, survival and incidence, is also essential to assess the impact of programmes. The community can also play a key role in designing and implementing data collection tools and analysing and interpreting findings. The publication on three interlinked patient monitoring systems (1) will also be updated to reflect this new monitoring and evaluation guidance. This will enable national programmes to document the effect of the shift in guidelines and can contribute to evaluating the impact of the guidelines. For each key area, potential topics to monitor and possible implications for revising monitoring systems are provided. Not all information needs to be captured routinely; data needs and the timing of data collection depend on the local context. Periodic evaluations and implementation research are also central to reviewing programmes. Social science and implementation research are important to assess perceptions and values of service recipients and communities along with barriers, facilitators and experiences in delivering and receiving services. Impact indicators, such as incidence, morbidity and mortality, are often diffcult to measure. Mathematical modelling is often undertaken to project various scenarios for programme planning and evaluating impact. Specifc data collection efforts and models for particular contexts may provide more accurate estimates. Drug resistance results in more rapid virological failure among people receiving first-line regimens and increases the need for second-line regimens, which may be associated with greater toxicity, adverse events, poorer adherence and higher costs. Algorithms for the 2013 recommendations for pregnant and breastfeeding women 234 Annex 4. Dosages of recommended antiretroviral drugs 242 230 Consolidated guidelines on the use of antiretroviral drugs for treating and preventing hiv infection 12. Adults and adolescentsa Children Clinical stage 1 Asymptomatic Asymptomatic Persistent generalized lymphadenopathy Persistent generalized lymphadenopathy Clinical stage 2 Moderate unexplained weight loss (<10% of Unexplained persistent hepatosplenomegaly presumed or measured body weight) Recurrent or chronic upper respiratory tract infections Recurrent respiratory tract infections (sinusitis, (otitis media, otorrhoea, sinusitis, tonsillitis) tonsillitis, otitis media, pharyngitis) Herpes zoster Herpes zoster Lineal gingival erythema Angular cheilitis Recurrent oral ulceration Recurrent oral ulceration Papular pruritic eruption Papular pruritic eruption Fungal nail infections Fungal nail infections Extensive wart virus infection Seborrhoeic dermatitis Extensive molluscum contagiosum Unexplained persistent parotid enlargement Clinical stage 3 Unexplained severe weight loss (>10% of Unexplained moderate malnutritionb not adequately presumed or measured body weight) responding to standard therapy Unexplained chronic diarrhoea for longer Unexplained persistent diarrhoea (14 days or more) than 1 month Unexplained persistent fever (above 37. For those aged less than 15 years, the clinical staging for children should be used. Decompensated cirrhosis is defined by the development of clinically evident complications of portal hypertension (ascites, variceal haemorrhage and hepatic encephalopathy) or liver insufficiency (jaundice). Annexes 233 234 Consolidated guidelines on the use of antiretroviral drugs for treating and preventing hiv infection Annex 3. Algorithm for early infant diagnosis 236 Consolidated guidelines on the use of antiretroviral drugs for treating and preventing hiv infection Annex 4. The duration of therapy with this drug should be limited to the shortest time possible. Countries planning for this transition, and those working to expand and strengthen their programme, may fnd it useful to refer to this readiness assessment checklist, which addresses a range of issues from national policy to facility readiness. When this simplifed weight-band dosing was developed, careful consideration was given to the usual body surface area of children from low- and middle-income countries in that weight band. The primary source of information for the guidance provided is the manufacturer’s package insert. This was supplemented with data from other clinical studies as well as expert paediatric pharmacology consultations. In some cases the dose for a component in a particular weight band may be somewhat above or below the target dose recommended by the manufacturer. This is inevitable given the limitations imposed by a fxed-dose combination, but care was taken to ensure that in no case would a child receive more than 25% above the maximum target dose or more than 5% below the minimum target dose. For simplifcation, Antiretroviral drugs that are no longer considered preferred or alternative options for children such as didanosine and saquinavir are no longer included in the dosing guidance. This dosing annex and the simplifed dosing schedule will be regularly reviewed and updated as further data or newer formulations become available, but national programmes are advised to consider the most recent product labelling for up-to-date information. Additional information can also be found in specifc drug information sheets in the Web Annex at www.
Immunization and Antibody Production : The immunogen 3-hemisuccinyloxyflurazepam generic cyklokapron 500mg otc symptoms 0f colon cancer, is emulsi- fied with complete Freund’s adjuvant 500 mg cyklokapron mastercard symptoms 11dpo. Subsequently order 500mg cyklokapron free shipping medications on nclex rn, booster injections of the thick-immunogen-emulsion-paste are administered after a span of 6-weeks cheap cyklokapron 500 mg with visa medicine 66 296 white round pill. The mono-as well as di-desethylmetabolites exhibited a cross-reactivity of 17 and 3. The resulting mixture is cooled to -30 °C and isoamyl nitrite in dioxane is added. The solution is stirred at – 30° to – 40 °C and aqueous ammonium sulfamate is added with continuous stirring. The chilled azide solution is added slowly, dropwise with constant vigorous stirring into a solution of bovine-serum albumin. The resulting pale-yellow solution is kept at 4°C for a duration of 36 hours and then dialysed against trimethamine buffer. After further dialysis for two days against distilled water, the immunogen is isolated by lyophilization. Immunization and Antibody Production : The lypphilized immunogen obtained above is dissolved in normal saline and emulsified with equal volumes of complete Freund’s adjuvant into a thick paste. Three New Zealand albino rabbits are immunized with the immunogen-paste through intradermal injections. The process is repeated twice at 2-weeks intervals followed by booster doses at monthly intervals. Specificity of Antibody binding of Chlordiazepoxide : A good number of benzodiazepines are tested for their ability to complete with labelled chlordiazepoxide for the respective antibody binding site. The mixture is incubated overnight at 4°C, and the protein-hapten complex is dialysed against distilled water thereby causing its purification. Six weeks after the initial does, booster doses are administered to the animals in each of their foot pads. Blood samples are collected 5-7 days after the booster injections and the serum is examined for antibodies to barbiturates. The antiserum is harvested when the serum antibody titer has attained its maximum level. It has been observed that while normal, rabbit serum failed to bind labelled phenobarbital, the serum from immunized rabbits bound 75 to 80% of the added pentobarbital and there exists a linear relationship between 14C-phenobarbital and the concentration of added antibody. Besides, when variable quantities of 14C- pentobarbital are added to a constant quantity of antibody, there exists a linear relationship between added and bound 14C-phenobarbital as depicted in Figure 32. Hence, there is a dire need for a sensitive method of plasma concentration evaluation which is satisfied by radioimmunoassay. The reaction mixture is dialysed exhaustively against normal saline to cause purification and the extent of conjugation is estimated by measuring the protein concen- tration**. The prepared emulsion is injected subcutaneously into four different sites in New Zealand albino rabbits. Six weeks after the initial injection, all the animals are placed on a regimen of weekly booster shots. The percentage inhibition is calculated and the values obtained from the first set of tubes is used to plot a standard curve. The concentrations of flunisolide from the standard curve values from their calulated percent- age inhibition value as depicted in figure 32. However, the isotope dilution method categorically makes a clear separation of the drug and its metabolites. Consequently, a non-specific antiserum is employed to actually quantify the total amount of both unlabelled and labelled substance present. Disadvantages : It has two main disadvantages : (i) The method is time-consuming, and (ii) It involves tiresome and meticulous process of isotope dilution. However, an intensive and extensive stereospecificity radioimmunoassay procedures have been adequately applied to a number of pharmaceutical substances since mid-seventies, for instance : atropine, propranolol, methadone-to name a few. Propranolol** which represents a comparatively better conceived example shall be discussed briefly as under with regard to its stereospecificity : Propranolol is a recemic mixture i. In actual practice, two antisersa have been developed experimentally, namely : (a) Antisera against the dl-racemic mixture, and (b) Antisera against the l-isomer (active form only). Thus, the concentrations of d-propranolol could be arrived at by subtracting the con- centration of l-isomer from the dl-mixture. It has been clearly demonstrated by Kawashima and coworkwers* that the d-propranolol undergoes distribution in vivo very sluggishly besides being metabolized more rapidly whereas the l-isomer gets distributed rather quickly to various tissues including the heart. Perhaps it could be possible that the stereospecificity of propranolol is caused due to the conformation of the drug-hapten in rela- tion to the carrier protein to a great extent, through this hypothesis remains to be ascertained scientifically. Consequently, the stabilization of the optical carbon by virtue of the conjugation to respective protein might improve upon the status of the specificity to a considerable extent. In order to prove the validity of this phenomenon one may carry out a definitive methodology whereby a closely monitored and controlled study of the antisera obtained by conjugates specifically prepared at the ‘asymmetric-carbon’ and at ‘another-site’ are both compared simultaneously under identical experimental parameters. Give structures, equations wherever applicable to strengthen your answer : (a) Morphine, (b) Hydromorphons and hydrocordone in Human Plasma, (c) Clonazepam, (d) Flurazepam in Human Plasma, (e) Chlordiazepoxide in Plasma, (f) Barbiturates, and (g) Flunisolide in Human Plasma. The water is not rapidly regained, but the compound should be kept in a desicator after drying and should be weighed quickly once it is removed. It will lose one H2O2 molecule at 100 °C and a second H2O2 molecule at approximately 130-140 °C and is difficult to dry to a constant weight. The wate is not rapidly regained but the compound should be kept in a desicator after drying and should be weighed quickly once it is removed. The following references were used in compiling these monographs: Fink, Mitchell et al. Medsafe Drug Data sheets (New Zealand Medicine and Medical Devices Safety Authority): http://www. Should any discrepancies exist between the printed & online versions, the latter should always take precedence. Paul • The monograph for recombinant Young for use in the Intensive Care Unit in Wellington Regional Hospital in 2011. Zealand dollars & are up to date as of February 2013 All doses have been checked independently by two Intensive Care Specialists. However if you suspect an Prices have been included to inform error, please check data with alternative prescribing choices where intravenous or sources and notify the editor. For example, the intravenous Clinical responsibility for the choice, preparation of Acetazolamide costs 250 dose, route & frequency of any times that of a single tablet medication always remains with the (bioavailability >90%). Administration Of Medicines Via Enteral Feeding Sodium Valproate 386 Tubes Sotalol 389 2. Each vial contains an amount of acetazolamide sodium equivalent to 500 mg of acetazolamide. Each 500-mg vial containing acetazolamide should be reconstituted with at least 5 ml of sterile water for injection prior to use. If, after an initial response, the patient fails to continue to diurese, do not increase the dose but allow for kidney recovery by skipping medication for a day. Acetazolamide yields best diuretic results when given on alternate days, or for 2 days alternating with a day of rest. Interference with the theophylline assay by acetazolamide depends on the solvent used in the extraction; acetazolamide may not interfere with other assay methods for theophylline.
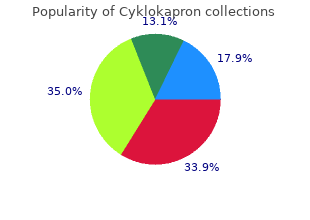
Cyklokapron 500mg lowest price. SHINEE maknae taemin photo.
Diseases
- Alopecia, epilepsy, pyorrhea, mental subnormality
- Arthrogryposis multiplex congenita neurogenic type
- Glucose-galactose malabsorption
- Coronary artery aneurysm
- Bellini Chiumello Rinoldi syndrome
- Acute lymphoblastic leukemia