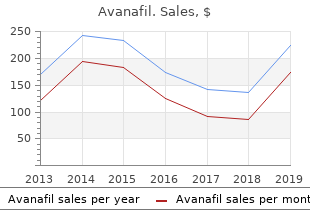
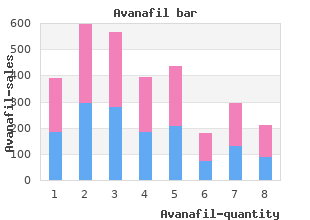
University of the South. W. Lee, MD: "Order Avanafil online - Proven Avanafil OTC".
Have the potential social purchase 200 mg avanafil with mastercard erectile dysfunction caused by low testosterone, cultural order avanafil 100 mg on-line erectile dysfunction vitamin d, and legal barriers that deter the meaningful participation of historically marginalized stakeholders been identifed and addressed? Transparency regarding the grounds for decisions Are the decision-making criteria transparent and is the rationale stated explicitly with reference to: Scientific evidence purchase avanafil 50 mg with amex impotence used in a sentence, including effectiveness and risk? Alignment between evidence and recommendations Are the recommendations appropriate for the epidemiological setting in which they will be implemented? Are the recommendations aligned with and do they support the implementation of the programme’s overarching vision discount avanafil 50mg overnight delivery erectile dysfunction pills pictures, goals and objectives? Has it been determined how such barriers will be dealt with and how the responses will affect programme planning? Key ethical principles of fairness, equity and urgency should also be observed in the process of reviewing and adapting guidelines. The design of effective and equitable policies implies that strategies should focus comprehensively on addressing barriers to access testing, prevention and treatment services, particularly those faced by key populations. Facility- and community-level reviews may be useful to understand the extent to which services are acceptable and adapted to the specifc needs of key populations. Impact is often a result of a complex set of factors and a combination of diverse inputs and activities or processes, and it is often not attributable to a single intervention or programme (5). Cost–effectiveness analysis is one of several economic evaluation tools used to measure the value of delivering particular services. Economic evaluation measures the costs and consequences of alternative programmes, which are then compared to assess how the greatest health benefts can be generated. As such, these programmes can contribute to overall cost–effectiveness, in addition to achieving other important objectives, such as reducing discrimination (18). These models also considered the relative cost–effectiveness of strategies, highlighting which of them, for a given budget, would be expected to maximize health gains. The modelling results should be interpreted in light of some important limitations. Moreover, the models did not consider how the estimated impact and cost–effectiveness of the various interventions would change if they were combined or only partly implemented. Models also did not address potential trade-offs with non-antiretroviral interventions, and several important issues were not covered, such as treatment of children. The benefts accrued from implementing them are likely to considerably outweigh the upfront investment needed and have the potential to fundamentally change the course of the epidemic. It is essential to design strategies to mitigate such events so that continued service delivery can be assured, especially for those most in need (20). Such considerations should not determine whether a particular recommendation is included or excluded from national guidelines but can be used as a tool to understand the impact of a recommendation and how best to adapt it and mobilize resources for its implementation. An implementation plan should clearly defne the set of activities required in a specifed period of time to achieve targeted outcomes, with a clear division of labour among all stakeholders involved in implementing programmes. Robust procurement and supply management systems are needed to ensure the continued availability of all necessary drugs, diagnostics and other commodities across the various levels of the health system. Pooled or joint procurement can be used to secure lower costs through economies of scale, and careful demand forecasting is key to minimizing waste. Fixed-dose combinations and once-daily therapy should be used whenever possible to support adherence and make treatment as convenient as possible for the people receiving therapy and their caregivers. Laboratory capacity must also be reviewed and services should be strengthened to cope with higher demand, and nationally standardized health information systems and patient monitoring tools should be used in all settings. Stronger interventions are also needed to maximize treatment adherence and retention across the continuum of care. Specifc interventions may be needed in particular settings, such as postpartum follow- up of mother–infant pairs. The quality of health care is a critical dimension to consider in the planning and adaptation process. The implementation of new guidelines provides an opportunity to comprehensively review and address such gaps. Critically, this requires effective monitoring and evaluation systems (see Chapter 11). A key component of sound quality assurance mechanisms is a clear delineation of roles and responsibilities for the delivery of the various functions and inputs (such as leadership, fnancing, supply chain management, human resources, monitoring and evaluation needed for effectively providing services at the national, regional, district, facility and individual clinician levels). Planning should also take into account the variety of providers involved in health service delivery, including public, private and not-for-proft organizations. Community involvement and peer outreach strategies are key to improve programme design, promote its sustainability and maximize coverage. Communication, leadership and advocacy Has it been determined who will be responsible for updating currently existing materials, including service delivery guidelines, protocols, clinical and laboratory standard operating procedures, monitoring and evaluation tools, patient monitoring mechanisms or systems, reference manuals, health worker training materials, job aids, supervisory checklists and materials for public information, education and communication? Has it been agreed who will take overall responsibility for advocacy with stakeholders such as political leaders, health personnel and the mass media? Staffing and human resources Has it been determined how many additional workers are required to implement new recommendations? Which cadres of health workers (physicians, health officers, nurses, midwifes, community health workers and laboratory assistants) are needed and how they can be recruited? Can task shifting and sharing be employed to optimize available human resources and expand service delivery? Has it been determined what systems are required for forecasting needs and procuring medicines and other commodities at the best possible prices? Has a transition plan been developed to phase out old medicines (such as d4T) and introduce new ones? Do supply management systems – especially at the peripheral level – need to be strengthened to manage increased demand? Is a regulatory process in place to approve and register new medicines and diagnostics in a timely manner? Are laboratory quality control and external quality assurance systems in place and fully functional? Do national laws allow for the purchase and importation of all necessary commodities? Do services need to be decentralized and/or integrated to support policy implementation? Infrastructure Has the necessary physical infrastructure (such as warehouses, meeting rooms, consultation space, laboratories, pharmacies, administration areas and equipment) and transport infrastructure (such as vehicles) needed to support implementation been identifed? Is additional communication infrastructure needed, including between health facilities, health workers, laboratories and clients? Costs Has the total annual investment of implementing new recommendations, including ancillary and other services, been estimated? Can potential cost-savings be achieved through economies of scale or synergies with other interventions and programmes? Monitoring and evaluation Does the monitoring and evaluation plan clearly identify the facility- and programme- level indicators needed to adequately monitor the coverage of interventions and impact of new recommendations? Have the human resources, equipment and infrastructure requirements been identified? Are monitoring and evaluation systems interoperable (between the local and central levels and among various donors) to avoid duplication and ensure consistency? Have the necessary quality control, quality assurance and quality improvement systems been identified and put in place to optimize service delivery?
Precautons Asthma; bronchiectasis; pulmonary emphysema or reduced pulmonary functon; use of excessive volume or too rapid administraton may result in lobar collapse; may interfere with thyroid-functon tests; important: because of risk of hypersensitvity reactons; adequate resuscitaton facilites must be immediately available when radiographic procedures are carried out cheap avanafil 200 mg mastercard does erectile dysfunction cause infertility. Adverse Efects Pyrexia; malaise; arthralgia; cough; occasionally; dyspnoea; atelectasis; pneu- monia; rarely buy cheap avanafil 50mg line erectile dysfunction medication for diabetes,; hypersensitvity reactons buy discount avanafil 50 mg online constipation causes erectile dysfunction. Dialysis Fluids Solutons for peritoneal dialysis are preparatons for intraperi- toneal use which contain electrolytes in a similar concentra- ton to that in plasma avanafil 100 mg discount erectile dysfunction obesity, and also contain glucose or another suitable osmotc agent. Peritoneal dialysis solutons always contain sodium, chloride, and hydrogen carbonate or a precursor; they may also contain calcium, magnesium, and potassium. In renal failure haemodialysis is the preferred method to correct the accumulaton of toxins, electrolytes and fuid. Peritoneal dialysis is less efcient than haemodialysis, but it is preferred in children, diabetc patents, and patents with unstable cardiovascular disease; it is also used in patents who can manage their conditon, or those who live far from a dialysis centre. In peritoneal dialysis, the soluton is infused into the peritoneal cavity, where exchange of electrolytes takes place by difusion and convecton, and excess fuid is removed by osmosis, using the peritoneal membrane as an osmotc membrane. The main complicaton of peritoneal dialysis is peritonits, which ofen results from poor exchange technique; infec- tons of the catheter exit site may also occur, again because of poor technique. With long-term dialysis progressive struc- tural changes to the peritoneal membrane occur, ultmately resultng in dialysis failure. Peritoneal dialysis It is preferred in children, diabetc patents, and patents with unstable cardiovascular disease; also used in patents who can manage their conditon, or those who live far from a dialysis centre. Contraindicatons Abdominal sepsis; previous abdominal surgery; severe infammatory bowel disease; pregnancy, excessive obesity; behavioural disturbances. Precautons Care required with technique to reduce risk of infecton; warm dialysis soluton to body temperature before use; some drugs may be removed by dialysis; generalised peritonits; traumatc abdominal lesions. Adverse Efects Infecton including peritonits; hernia; haemoperitoneum; hyperglycaemia, protein malnutriton; blocked catheter; fuid and electrolyte imbalance, disequilibrium syndrome, muscle cramp. Treatment should be started early in the course of the disease, before joint damage starts. Because long-term therapy can result in retnopathy ophthalmolog- ical examinatons should be conducted before and during treatment. Adverse reactons include blood disorders (bone marrow suppres- sion), hepatotoxicity, skin reactons and gastrointestnal distur- bances. Penicillamine is not a frst-line drug and its use is limited by a signifcant incidence of adverse efects including blood disor- ders (bone marrow suppression), proteinuria and rash. Cortcosteroids are potent ant-infammatory drugs but their place in the treatment of rheumatoid arthrits remains contro- versial. Their usefulness is limited by adverse efects and their use should be controlled by specialists. Cortcosteroids are usually reserved for use in patents with severe disease which has failed to respond to other antrheumatc drugs, or where there are severe extra-artcular efects such as vascu- lits. Although cortcosteroids are associated with bone loss this appears to be dose-related; recent studies have suggested that a low dose of a cortcos- teroid started during the frst two years of moderate to severe rheumatoid arthrits may reduce the rate of joint destruc- ton. Relatvely high doses of a cortcosteroid, with cyclophosphamide, may be needed to control vasculits. Elderly over 60 years- Rheumatoid arthrits and ankylosing spondylits: 300 mg twice daily. Precautons Monitor throughout treatment including blood counts; hepatc impairment (Appendix 7a); renal impairment; elderly (reduce dose); lactaton (Appendix 7b); interactons (Appendix 6c, 6d); pregnancy (Appendix 7c). Patents should be warned to report immediately any signs or symptoms of bone marrow suppression; for example unexplained bruising or bleeding; purpura; infecton; sore throat. Adverse Efects Hypersensitvity reactons requiring immediate and permanent withdrawal include malaise; dizziness; vomitng; diarrhoea; fever; rigors; myalgia; arthralgia; rash; hypotension and intersttal nephrits; dose-related bone marrow suppression; liver impairment; cholestatc jaundice; hair loss and increased suceptbility to infectons and colits in patents also receiving cortcosteroids; nausea; rarely, pancreatts and pneumonits. Dose Oral Acute rheumatoid arthrits including juvenile idiopathic arthrits: 150 mg/day (max. Lefunomide* Pregnancy Category-X Schedule H Indicatons Actve rheumatoid arthrits, psoriatc arthrits. Dose Oral Actve rheumatoid arthrits: Adults- 100 mg once daily as loading dose for 3 days. Precautons Liver disease, kidney disease, heart disease, women of child bearing age, monitor blood counts and blood pressure regularly. Adverse Efects Diarrhoea occurs in approximately 25% of patents, other adverse efect associated are mild alopecia, weight gain, increased blood pressure. Intramuscular, subcutaneous or intravenous route in severe atack under expert medical supervision at a dose of 7. Contraindicatons Lactaton (Appendix 7b); pregnancy (Appendix 7c); immunodefciency syndromes; signifcant pleural efusion or ascites. Patents should be warned to report immedi- ately any signs or symptoms of bone marrow suppression; for example unexplained bruising or bleeding; purpura; infecton; sore throat. Adverse Efects Blood disorders (bone marrow suppression); liver damage; pulmonary toxicity; gastrointestnal disturbances-if stomatts and diarrhoea occur; stop treatment; renal failure; skin reactons; alopecia; osteoporosis; arthralgia; myalgia; ocular irritaton; precipitaton of diabetes. Dose Oral Adult- Severe actve rheumatoid arthrits: initally 125 to 250 mg daily for one month, increased by increments of similar amount at intervals of not less than 4 weeks to usual maintenance dose of 500 to 750 mg daily in divided doses (max 1. If remission sustained for 6 months, reduce daily dose (125 to 150 mg every 12week may be atempted). Elderly- Severe actve rheumatoid arthrits: initally usual 125 mg daily for 1 month. Increase by increments of similar amount at intervals of not less than 4 weeks (max. Child- Severe actve rheumatoid arthrits: maintenance dose of 15 to 20 mg/kg daily, inital amount at intervals of 4 weeks over a period of 3 to 6 months. Precautons Monitor throughout treatment including blood counts and urine tests; renal impairment; avoid concurrent gold; chloroquine or immunosuppressive treatment; avoid oral iron within 2 h of a dose. Patents should be warned to report immediately any signs or symptoms of bone marrow suppression; for example unexplained bruising or bleeding; purpura; infecton; sore throat. Adverse Efects Initally nausea (less of a problem if taken before food or on retring; and if inital dose is only gradually increased); anorexia; fever; taste loss (mineral supplements not recommended); blood disorders including thrombocytopenia; neutropenia; agranulocytosis and aplastc anaemia; proteinuria; rarely, haematuria (withdraw immediately); haemolytc anaemia; nephrotc syndrome; lupus erythematosus- like syndrome; myasthenia-like syndrome; polymyosits (rarely, with cardiac involvement); dermatomyosits; mouth ulcers; stomatts; alopecia; bronchiolits and pneumonits; pemphigus; glomerulonephrits (Goodpasture syndrome) and erythema multforme (Stevens-Johnson syndrome); male and female breast enlargement; rash (early rash disappears on withdrawing treatment-reintroduce at lower dose and increase gradually; late rash is more resistant- either reduce dose or withdraw treatment). Dose Oral Acute rheumatoid arthrits: Adult- initally 500 mg daily increase by 500 mg at interval of one week (max. Contraindicatons Hypersensitvity to salicylates and sulfonamides; severe renal impairment; child under 2 years; porphyria. Patents should be warned to report immediately any signs or symptoms of bone marrow suppression; for example unexplained bruising or bleeding; purpura; infecton; sore throat. Adverse Efects Nausea; diarrhoea; headache; loss of appe- tte; fever; blood disorders (including Heinz body anaemia; megaloblastc anaemia; leu- kopenia; neutropenia; thrombocytopenia); hypersensitvity reactons (including rash; urt- caria; erythema multforme (Stevens-Johnson syndrome); exfoliatve dermatts; epidermal necrolysis; pruritus; photosensitzaton; ana- phylaxis; serum sickness; intersttal nephrits; lupus erythematosus-like syndrome); lung complicatons (including eosinophilia; fbros- ing alveolits); ocular complicatons (includ- ing periorbital oedema); stomatts; parotts; ataxia; aseptc meningits; vertgo; tnnitus; alopecia; peripheral neuropathy; insomnia; depression; hallucinatons; kidney reactons (including proteinuria; crystalluria; haematu- ria); oligospermia; rarely, acute pancreatts; hepatts; urine may be coloured orange. Salicylates, including acetylsalicylic acid are also not suitable because they may increase plasma-urate concentra- tons. It does not induce fuid retenton and can there- fore be given to patents with heart failure; it can also be given to patents receiving antcoagulants. Chronic Gout: For long-term control of gout in patents who have frequent atacks, the xanthine oxidase inhibitor allopurinol may be used to reduce producton of uric acid. It should not be used to treat an acute atack since it may prolong it indefnitely.
Master of the Wood (Sweet Woodruff). Avanafil.
- Preventing and treating lung, stomach, liver, gallbladder, and urinary disorders; nervousness; hemorrhoids; sleeplessness; migraines; skin problems; and other conditions.
- Are there safety concerns?
- What is Sweet Woodruff?
- How does Sweet Woodruff work?
- Dosing considerations for Sweet Woodruff.
Source: http://www.rxlist.com/script/main/art.asp?articlekey=96113
Synthetic nano-low density lipoprotein as targeted drug delivery vehicle for glioblastoma multiforme cheap avanafil 100mg amex impotence age 45. Novel gelatin–siloxane nanoparticles decorated by Tat peptide as vectors for gene therapy generic 50 mg avanafil fast delivery impotence early 30s. Nanostructured nanophosphates for non viral gene deliv- ery: Influence of the synthesis parameters on transfection efficiency cheap avanafil 100 mg with amex erectile dysfunction treatment atlanta ga. A novel nanocapsule delivery system to overcome intestinal degradation and drug transport limited absorption of P glycoprotein substrate drug cheap avanafil 100 mg with visa impotence gandhi. Quality by design: Understanding the product variability of a self-nanoemulsifying drug delivery system of cyclosporine A. Enhanced intratumoral uptake of quantum dots con- cealed within hydrogel nanoparticles. Drug release characteristics of physically cross- linked thermosensitive poly(N-vinyl caprolactum) hydrogel particles. Smart nanoprobes for ultrasensitive detection of breast cancer via magnetic resonance imaging. Core/shell nanoparticles for multiple biological detection with enhanced sensitivity and kinetics. Nanotube antibody biosensor arrays for the detection of circulating breast cancer cells. Pharmacokinetics and in vivo drug release rates in liposomal nanocarrier development. Nanoparticulate systems for nasal delivery of drugs: A real improvement over simple systems. Dendrimers as drug carriers: Applications in different routes of drug administration. Development, characterization and toxicity evalua- tion of amphotericin B-loaded gelatin nanoparticles. Electrostatic ligand coatings of nanoparticles enable ligand-specific gene delivery to human primary cells. Polymeric Nanoparticles for Small-Molecule Drugs: Biodegradation of Polymers and Fabrication of Nanoparticles Sheetal R. Das Department of Pharmaceutical Sciences, Butler University, Indianapolis, Indiana, U. Currently, small-molecule drugs continue to dominate the pharmaceutical market despite biotech drugs making a distinct niche for themselves, because the former enjoys the advantages of small molecular size, solubility, and permeability, which are favorable for passive membrane diffusion. However, the perspective of a drug as a chemical compound used for the prevention, diagnosis, and treatment of a disease state has changed drastically over the past couple of decades as we learn that the mode of delivery of a drug could radically change the therapeutic outcomes of a disease state. For instance, the entrapment of a molecule in a nanoparticulate system could pave the way for better cellular uptake or drug targeting to specific tissues for those drugs with poor bioavailability, in addition to providing prolonged drug release effects. Polymeric nanoparticles are defined as colloidal particles ranging between 10 and 1000 nm in size and composed of natural or artificial polymers (1). Since the diameter of the smallest capillaries in the human body is about 4 m, in order for the nanoparticles to access all locations in the body by the intravenous, intra- muscular, or subcutaneous route, the solid particles should preferably have a small diameter (2). The small particle size also reduces potential irritant reactions at the injection site (3). The utility of a nanoparticle delivery system is dependent upon the bioacceptability of the carrier polymer, which, in turn, is affected by the parti- cle size and physicochemical properties of the polymer. Ultimately, the bioaccept- ability of the polymer, physicochemical properties of the drug, and the therapeutic goal will influence the final choice of the appropriate polymer, particle size, and the manufacturing method. Based on the manufacturing method for the nanopar- ticles, drug molecules can be either dissolved in a liquid core or dispersed within a dense polymeric matrix, resulting in nanocapsules or nanospheres. Liposomes, niosomes, and microemulsions are similar to polymeric nanoparticles with respect to their shape, size, and mode of administration, and thus serve as alternative modes of novel colloidal drug carrier systems. However, nanoparticles offer addi- tional advantages when compared with the other colloidal carriers, such as higher stability when in contact with biological fluids, high drug-loading capacities, and protection by the solid matrix of the incorporated drug against degradation, thus leading to increased intracellular concentration of the drug (4). Also, because of 16 Polymeric Nanoparticles for Small-Molecule Drugs 17 their polymeric nature, controlled drug release can be obtained with nanopar- ticles. The surface of the polymeric nanoparticles can be covalently conjugated to folic acid, monoclonal antibodies, and aptamers to achieve targeted delivery and cell-specific uptake. While many reports have demonstrated the increase in solubility of poorly soluble small molecules by modifying the shape, size, and functional groups present on the molecule, as well as the increase in permeability by the incorporation of lipid components into the drug, the entrapment of drugs in nanoparticles offers opportunities for the modulation of both solubility and per- meability. They can be fabricated into various shapes and sizes, with tailored pore morphologies, mechanical properties, and degradation kinetics to suit a variety of applications. By selecting the appropri- ate polymer type, molecular weight, and copolymer blend ratio, the degradation/ erosion rate of the nanoparticles can be controlled to achieve the desired type and rate of release of the encapsulated drug. The common biodegradable poly- mers used in drug delivery include (i) polyesters, such as lactide and glycolide copolymers, polycaprolactones, poly( -hydroxybutyrates), (ii) polyamides, which includes natural polymers such as collagen, gelatin, and albumin, and semisyn- thetic pseudo-poly(amino acids) such as poly(N-palmitoyl hydroxyproline ester), 18 D’Mello et al. Owing to the presence of methyl groups in the lactide polymers, they are more hydropho- bic than the glycolide polymers. Also, the water uptake increases as the glycolide ratio in the copolymer increases (11). The transition glass tem- peratures of the copolymers range from 36◦C to about 67◦C. Furthermore, hydrolysis is enhanced by the accumu- lation of acidic products and the reduction of pH facilitated by the carboxylic acid end groups, which is an autocatalytic degradation process (13–15). The degrada- tion of these polymers differs in vivo and in vitro, mainly because, although in vivo there is no major influence of enzymes during the glassy state of the polymer, these enzymes can play a significant role when the polymer becomes rubbery (15). Nor- mally, 50:50 lactide/glycolide copolymers have the fastest half-life of degradation, around 50 to 60 days, whereas 65:35, 75:25, and 85:15 lactide/glycolide copolymers have progressively longer degradation half-lives in vivo. The half-life of these linear polyesters can be increased by coblending with more hydrophobic comonomers such as polycaprolactone. Biocompatibility The evaluation of the biocompatibility of biodegradable polymers takes into con- sideration the incidence of the inflammatory and healing responses of the injected and implanted materials. The particles after an intramuscular or a subcutaneous injection usually have a high surface area/low volume ratio within a given tissue volume. Table 1 outlines the tissue responses to the polymer materials that are divided into three time phases (10). Nanoparticles, when given intravenously, can modulate the inflammatory and healing responses in their presence. These responses are usually lesser in mag- nitude because the particles are present as single, isolated particles and not in groups and also because the cellular injury at the site of the particle is minimal (21). By modifying the polymer type, the copolymer composition, the polymer molecular weight, and the porosity of the microspheres, their degradation rate can be varied from days to months. It undergoes bulk erosion by random hydrolytic chain cleavage in the first phase, resulting in a decrease in the molecular weight of the polymer. This is followed by the second phase, in which these low molecular weight fragments undergo phagocytosis or solubilization in the body fluids. Their degradation rate can be enhanced by the addition of additives such as oleic acid and tertiary amines, which act as catalysts in the chain hydrolysis process.
For those products that are unlikely to be removed acceptance criteria for the product would not be consid- from the immediate container order avanafil 50mg free shipping impotence 60784, such as creams or oint- ered acceptable change best 100 mg avanafil erectile dysfunction drug companies. This is a stress test designed to ments in tubes dispensed directly to the patient and oph- determine the intrinsic photostability characteristics of thalmic products cheap avanafil 100mg erectile dysfunction drugs at cvs, the use of a labeling storage statement new drug substances and products discount 100mg avanafil visa erectile dysfunction caused by vasectomy, and no correlation regarding light is optional. For products that may be has been developed to equate a within-specification removed from the immediate pack, such as pharmacy result to an expiration dating period. Change after Exposure in the Market Pack change as acceptable because the processes may be inde- pendent and additive. For example, a 5% loss in potency If changes that are observed are acceptable only when the caused by photodegradation may be considered accept- product in the market pack is exposed under the conditions able if that is the only type of degradation observed. If described in the Q1B guidance, labeling storage state- the product is also expected to degrade 5% over the shelf ments regarding light should be included. Quantitative analysis of the color change is not recommended, as these changes are not likely to occur When degradation products are detected upon storage, the under actual storage conditions. In the absence of change following information about them should be submitted: 54 Handbook of Pharmaceutical Manufacturing Formulations: Semisolid Products • Procedure for isolation and purification M. A satisfactory inspection of the laboratory or lab- • Biological effect and pharmacological actions, oratories that will perform the testing will be necessary. Time frames should be If racemization of the drug substance in the dosage form established to encompass the date of production, date of is possible, the information described above also should quality control release of the dosage form, bulk packaging, be provided. Maximum time frames for each operation should be estab- A study of the effects of temperature variation, particu- lished and substantiated by the applicant. However, biotechnologi- • temperature cycling study for drug products cal and biological products have distinguishing characteris- that may be exposed to temperature variations tics to which consideration should be given in any well- above freezing may consist of three cycles of 2 defined testing program designed to confirm their stability days at refrigerated temperature (2°–8°C) fol- during the intended storage period. For such products in lowed by 2 days under accelerated storage con- which the active components are typically proteins or ditions (40°C). The products are particularly sensi- tures may consist of three cycles of 2 days at tive to environmental factors such as temperature changes, freezer temperature (−10° to −20°C) followed oxidation, light, ionic content, and shear. To ensure mainte- by 2 days under accelerated storage conditions nance of biological activity and to avoid degradation, strin- (40°C). With these concerns in mind, the applicant should • Alternatives to these conditions may be accept- develop the proper supporting stability data for a bio- able with appropriate justification. Primary data to support a requested is submitted to the agency, with a commitment to place storage period for either drug substance or drug product the first three manufacturing-scale batches into the long- should be based on long-term, real-time, real-condition term stability program after approval. Thus, the development of a proper long- The quality of the batches of drug substance placed term stability program becomes critical to the successful into the stability program should be representative of the development of a commercial product. The purpose of this quality of the material used in preclinical and clinical document is to give guidance to applicants regarding the studies and of the quality of the material to be made at type of stability studies that should be provided in support manufacturing scale. It is understood that during the material) made at pilot-scale should be produced by a review and evaluation process, continuing updates of ini- process and stored under conditions representative of tial stability data may occur. Containers of The guidance in this section applies to well-characterized reduced size may be acceptable for drug substance stabil- proteins and polypeptides, their derivatives, and products ity testing provided that they are constructed of the same of which they are components and that are isolated from material and use the same type of container and closure tissues, body fluids, or cell cultures or produced using system that is intended to be used during manufacture. Intermediates stability data for products such as cytokines (interferons, During manufacture of biotechnological and biological interleukins, colony-stimulating factors, tumor necrosis products, the quality and control of certain intermediates factors), erythropoietins, plasminogen activators, blood may be critical to the production of the final product. In plasma factors, growth hormones and growth factors, insu- general, the manufacturer should identify intermediates lins, monoclonal antibodies, and vaccines consisting of and generate in-house data and process limits that ensure well-characterized proteins or polypeptides. Drug Product (Final Container Product) Stability information should be provided on at least three 3. However, because manufacturers ble, batches of final container product included in stability of biotechnological and biological products sometimes testing should be derived from different batches of bulk use traditional terminology, traditional terms are specified material. Product Where bulk material is to be stored after manufacture, but expiration dating should be based on the actual data sub- before formulation and final manufacturing, stability data mitted in support of the application. Because dating is should be provided on at least three batches for which based on the real-time/real-temperature data submitted for manufacture and storage are representative of the manu- review, continuing updates of initial stability data should facturing scale of production. The qual- stability data at the time of submission should be submit- ity of the final container product placed on stability studies ted in cases where storage periods greater than 6 months should be representative of the quality of the material used are requested. Data from pilot- of less than 6 months, the minimum amount of stability scale batches of drug product may be provided at the time data in the initial submission should be determined on a the application is submitted to the agency, with a com- case-by-case basis. Data from pilot-scale batches of drug mitment to place the first three manufacturing-scale substance produced at a reduced scale of fermentation and batches into the long-term stability program after approval. Protocol lish the dating for a product, and in the event that the The marketing application should include a detailed pro- product produced at manufacturing scale does not meet tocol for the assessment of the stability of both drug those long-term stability specifications throughout the dat- substance and drug product in support of the proposed ing period or is not representative of the material used in storage conditions and expiration dating periods. Sample Selection ing period including, for example, well-defined specifi- cations and test intervals. When the intended use of a product is linked to a defin- Matrixing—the statistical design of a stability study able and measurable biological activity, testing for in which different fractions of samples are tested at dif- potency should be part of the stability studies. For the ferent sampling points—should be applied only when purpose of stability testing of the products described in appropriate documentation is provided that confirms that this guidance, potency is the specific ability or capacity the stability of the samples tested represents the stability of a product to achieve its intended effect. The differences in the samples for the same the measurement of some attribute of the product and is drug product should be identified as, for example, cover- determined by a suitable in vivo or in vitro quantitative ing different batches, different strengths, different sizes of method. In general, potencies of biotechnological and the same closure, and possibly, in some cases, different biological products tested by different laboratories can container and closure systems. Matrixing should not be be compared in a meaningful way only if they are applied to samples with differences that may affect stabil- expressed in relation to that of an appropriate reference ity, such as different strengths and different containers and material. For that purpose, a reference material calibrated closures, where it cannot be confirmed that the products directly or indirectly against the corresponding national respond similarly under storage conditions. The design of a protocol that incorporates brated, whenever possible, against nationally or interna- bracketing assumes that the stability of the intermediate tionally recognized standards. Where no national or inter- condition samples are represented by those at the national reference standards exist, the assay results may extremes. In certain cases, data may be needed to demon- be reported in in-house derived units using a characterized strate that all samples are properly represented by data reference material. Dissociation of the active ingredient or or parameter that profiles the stability characteristics of a ingredients from the carrier used in conjugates or adju- biotechnological or biological product. As a consequence, vants should be examined in real-time/real-temperature the manufacturer should propose a stability-indicating studies (including conditions encountered during ship- profile that provides assurance that changes in the identity, ment). The assessment of the stability of such products purity, and potency of the product will be detected. The items emphasized in the follow- active compound from the second moiety, in vivo ing subsections are not intended to be all-inclusive, but assays) or the use of an appropriate surrogate test should represent product characteristics that should typically be be considered to overcome the inadequacies of in vitro documented to demonstrate product stability adequately. Purity and Molecular Characterization powders or lyophilized cakes, pH, and moisture For the purpose of stability testing the products described level of powders and lyophilized products. If there is any indi- of stability testing, tests for purity should focus on meth- cation during preliminary stability studies that ods for determination of degradation products. Limits of • The container/closure has the potential to affect acceptable degradation should be derived from the analyt- the product adversely and should be carefully ical profiles of batches of the drug substance and drug evaluated (see following). Temperature comprehensive characterization of the drug substance or drug product (e. As examples, meth- bility studies may be confined to the proposed storage ods that may contribute to this include electrophoresis temperature. Therefore, where it can be demonstrated that the proposed Wherever significant qualitative or quantitative containers (and conditions of storage) afford sufficient changes indicative of degradation product formation are protection against high and low humidity, stability tests at detected during long-term, accelerated, or stress–stability different relative humidities can usually be omitted.