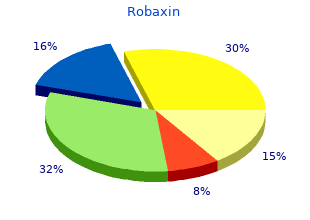
Virginia Intermont College. M. Milok, MD: "Order online Robaxin cheap no RX - Trusted online Robaxin OTC".
B-cell receptor a phase 1 study of CAL-101 best 500mg robaxin spasms while pregnant, An isoform-selective inhibitor of triggers drug sensitivity of primary CLL cells by controlling phosphatidylinositol 3-kinase P110 500mg robaxin spasms down legs when upright, in patients with glucosylation of ceramides robaxin 500mg spasms meaning. Oncogenically active (ASH Annual Meeting Abstracts) discount robaxin 500 mg visa muscle relaxant commercial. Exploiting synthetic demonstrates clinical activity and pharmacodynamic effects in lethality for the therapy of ABC diffuse large B cell lymphoma. Bruton’s tyrosine kinase (BTK) inhibitor PCI-32765 with 35. Chemokines and chemokine receptors in chronic bendamustine (B)/rituximab (R) (BR) in patients (pts) with lymphocytic leukemia (CLL): from understanding the basics relapsed/refractory (R/R) chronic lymphocytic leukemia (CLL): towards therapeutic targeting. A phase I trial of the tic strategy for chronic lymphocytic leukemia by combining Bruton’s tyrosine kinase (BTK) inhibitor, ibrutinib (PCI- GS-1101, a PI3 kinase delta (PI3K) inhibitor and a novel 32765), in combination with rituximab (R) and bendamustine in highly selective spleen tyrosine kinase (Syk) inhibitor, GS- patients with relapsed/refractory non-Hodgkin’s lymphoma 9973 [abstract]. A phase I/II study of evaluating activity and tolerability of BTK inhibitor PCI-32765 the selective phosphatidylinositol 3-kinase-delta (PI3K) and ofatumumab in patients with chronic lymphocytic leukemia/ inhibitor, GS-1101 (CAL-101), with ofatumumab in patients with small lymphocytic lymphoma (CLL/SLL) and related diseases. Younes A, Flinn I, Berdeja J, Friedberg J, Alberti S. Phase 1b plasma levels and the risk for disease progression in chronic study combining ibrutinib with rituximab, cyclophosphamide, lymphocytic leukemia. Paraffin-based 6-gene with CD20-positive B-cell non-Hodgkin lymphoma (NHL). Beyond JAK2 V617F, a more detailed picture of the pathobiologic basis for activated JAK-STAT signaling has emerged. In some patients with myelofibrosis (MF), next-generation sequencing technologies have revealed a complex clonal architecture affecting both genetic and epigenetic regulators of cell growth and differentiation. Although these bench-top findings have informed the clinical development of JAK inhibitors in MF, they have also provided scientific context for some of their limitations. The JAK1/JAK2 inhibitor ruxolitinib is approved for treatment of MF in North America and Europe and other lead JAK inhibitors discussed herein (fedratinib [SAR302503], momelotinib [CYT387], and pacritinib [SB1518]), have entered advanced phases of trial investigation. Uniformly, these agents share the ability to reduce spleen size and symptom burden. A major challenge for practitioners is how to optimize dosing of these agents to secure clinically relevant and durable benefits while minimizing myelosuppression. Suboptimal responses have spurred a “return to the bench” to characterize the basis for disease persistence and to inform new avenues of drug therapy. Lessons from the bench mutations in LNK or CBL ( 5% frequency)15,16 or abrogation of Normal hematopoiesis relies on the cytoplasmic tyrosine kinase suppressor of cytokine signaling (SOCS) activity (due to SOCS gene JAK2, which associates with the type I cytokine receptors for hypermethylation or increased protein phosphorylation) provide alter- erythropoietin, thrombopoietin, and G-CSF. Initial models predicted that logic pathways is common in MPN (Table 1)6,22 and contributes to the mutation relieves inhibition of the kinase (JH1) domain by the disease initiation and progression. Such modeling information is critical to the development chronic myeloid leukemia (CML), BCR-ABL1 is sufficient as a of next-generation mutant-specific inhibitors, which may exhibit genetic driver and has served as a paradigmatic target of better targeting of the malignant clone with less suppression of successful therapy with ABL tyrosine kinase inhibitors. Abnormalities in ment for cytokine receptor binding by ligand. Although activation of epigenetic pathways that control DNA methylation and modifica- the JAK-STAT pathway is common in myeloproliferative neoplasms tion of chromatin (detailed by Abdel-Wahab24) and mutations in (MPNs), it cannot be solely explained by the V617F or exon 12 JAK2 spliceosome factors have also emerged as important aspects of mutations that are found in 1% to 3% of PV patients. For example, transmembrane domain mutations (eg, W515 L/K) in MPL,13,14 the receptor for thrombopoietin, are found Among the MPNs, MF is notoriously characterized by a patho- in 1% to 5% of patients with ET and in 5% to 10% of PMF patients and logic milieu of proinflammatory and profibrotic cytokines (eg, lead to cytokine-independent growth and constitutive JAK-STAT IL-6, basic FGF, TGF- , TNF- , hepatocyte growth factor, signaling. Impaired negative regulation of JAK-STAT signaling due to VEGF, and others). Mutation frequency in chronic-phase and post-MPN AML cades and V617F-bearing neoplastic cells is a rational applica- tion of these drugs. In this regard, cytokine down-modulation by ruxolitinib25 and fedratinib27 has been demonstrated. It has also been shown that cytokines secreted by BM stromal cells protect MPN clones from JAK2 inhibitor treatment,28 highlighting the need to target both the BM microenvironment and malignant clone. Lessons from the bedside In MF, the decision regarding when and what type of therapy to initiate depends on a nexus of factors related to the patient, disease stage,29 primary clinical manifestations, drug-specific consider- ations, and treatment goals (Figure 1). Similarly, after treatment is initiated, a nuanced understanding of the relationship among drug dose, hematologic and nonhematologic adverse events, and efficacy outcomes is critical to optimizing patient care. The experience with JAK inhibitors highlights many of these interacting issues. The extramedullary hematopoiesis associated with MF-related constitutional symptoms and disease-related ca- splenomegaly is a cardinal feature of MF and is linked to abdominal chexia. Increased cytokine blood levels have been correlated pain and impairment of quality of life (QOL). A 35% reduction in with transfusion dependence, increased splenomegaly, thrombo- spleen volume by CT/MRI has been validated as a radiographic cytopenia, and shortened survival. Pre- and on-treatment considerations with use of JAK inhibitors in MF. Studies of lead JAK inhibitors in patients with MF the primary end point of a 35% reduction in spleen volume at vassed the unique spectrum of symptoms afflicting MF patients. In COMFORT-II, at week 48 (primary end point) and constitutional symptoms was achieved in the majority of sub- week 24 (key secondary end point), 28. Among the ruxolitinib-treated patients who achieved at COMFORT-I, a significantly higher proportion of patients in the least a 35% reduction in spleen volume in the COMFORT studies, ruxolitinib arm compared with the placebo group reported a 50% 60% of patients maintained this response with follow-up ranging reduction in the TSS from baseline to week 24 (45. Patients receiving ruxolitinib had a mean improvement hoc analysis of the COMFORT-I trial revealed that crossover of 46. Improvement in each patients originally randomized to ruxolitinib because of the interval individual symptom was observed, whereas all symptoms worsened spleen growth while they were receiving placebo before starting in the placebo group. Improvements in symptoms and QOL were of these drugs will help better define their comparative effects on the sustained with 96 weeks of follow-up. Conventional agents such as hydroxyurea, immuno- modulatory drugs (eg, thalidomide, lenalidomide, and pomalido- mide) with or without corticosteroids, androgens, and erythropoiesis- Impact of JAK2 V617F mutation status on response stimulating agents have shown minimal effects in alleviating Given the successful application of ABL-tyrosine kinase inhibitors MF-related fatigue or other constitutional symptoms. In addition, in CML, it is not surprising that some maintain the perception that until the development of the MF symptom assessment form,39 no JAK2 inhibitors, as an iteration of “targeted therapy,” only benefit instrument of patient reported outcomes (PRO) effectively can- patients with the JAK2 V617F mutation. However, JAK inhibitors Hematology 2013 531 Table 3. Efficacy and safety data for lead JAK inhibitors in patients with MF PPV/PET MF indicates post-polycythemic/post-ET MF; RUX, ruxolitinib; PBO, placebo; TSS, total symptom score; IWG-MRT, International Working Group for Myeloproliferative NeoplasmsResearchandTreatment;AE,adverseevent;DLT,dose-limitingtoxicity;ALT,alanineaminotransferase;AST,aspartateaminotransferase;MTD,maximumtolerateddose; EORTC,EuropeanOrganizationforResearchandTreatmentofCancer;andFact-Lym,FunctionalAssessmentofCancerTherapy-Lymphoma. In COMFORT-I, the mean changes in doses ranging from 10 to 25 mg BID. For example, a “start low and was 33% versus 14% in ruxolitinib-treated V617F-positive and escalate” rather than “start high and de-escalate” algorithm may be V617F-negative subgroups, respectively. Dose-response relationships Managing anemia and thrombocytopenia In COMFORT-I and COMFORT-II, a minimum platelet count of Anemia and thrombocytopenia are expected “on-target” effects of 100 109/L was required for eligibility, and starting doses of JAK2 inhibition because of the dependence of both erythropoietin ruxolitinib depended on the platelet count at baseline (100- and thrombopoietin receptors on signaling via the JAK2 tyrosine 200 109/L: 15 mg twice daily [BID]; 200 109/L: 20 mg kinase. In the COMFORT-I and COMFORT-II studies, anemia and BID). In COMFORT-I, 70% of patients underwent predefined thrombocytopenia (including grade 3/4 events) were more common dose adjustments during the first 12 weeks of therapy (primarily for with ruxolitinib (Table 1) than with placebo or BAT and typically cytopenias). Doses of 10 mg BID and higher and, by week 24, increased to a higher steady state that was closer to were associated with a more robust level of treatment benefit.
Current JAK inhibitor clinical trials for patients with an MPN JAK inhibitor Second Class second Trial number [target(s)] agent agent Disease (www quality robaxin 500mg spasms meaning in urdu. Further validation of this with the greatest toxicity observed in patients receiving upfront approach is ongoing robaxin 500 mg on line muscle relaxant with ibuprofen. Imetelstat is currently on hold for use in ET and PV due to vascular events in PV is a crucial goal and is accomplished by liver toxicity quality robaxin 500 mg muscle relaxant 4212. Immunomodulatory agents have proven efficacious controlling hematocrit levels to 45% and using low-dose aspi- in the treatment of anemia and thrombocytopenia buy 500mg robaxin with amex spasms calf. The positive impact of JAK2 inhibitors on splenomegaly, symp- toms, performance status, and quality of life makes these therapies a Selection of PV or ET patients needing cytoreductive therapy logical adjunct to allo-SCT, particularly as preconditioning. The use should incorporate both the MPN risk score and clinical scenario of ruxolitinib in allo-HSCT is currently under prospective investiga- (ELN recommendations) such as intolerance of phlebotomy. Regular assessment with MPN-10 may be briefly halted due to the development of tumor lysis syndrome and beneficial in determining which patients are symptomatically under- adverse events, but has resumed accrual based on interim analysis. For both ET and PV, the presence of former or current A German allo-SCT trial similarly structured to the French study vascular events automatically constitutes a high-risk disease has demonstrated improvements in constitutional symptoms in 86% profile and frontline cytoreductive therapy should be used. In of patients receiving ruxolitinib at the time of transplantation, with concordance with ELN recommendations, HU is frontline therapy 45% displaying a 50% reduction in spleen size. Clinical trials may also be consid- done in the setting of a clinical trial such as the MPD-RC 114 trial ered. In our opinion, high-risk patients who, despite receiving (www. Data from the randomized PV and ET phase 3 trials of ruxolitinib for PV may lead to US Food and As previously discussed, in 2014, the management of PV and ET Drug Administration (FDA) or European Medicines Agency patients is primarily focused on prevention of thrombohemorrhagic (EMA) approval and provide a clear second-line therapy for PV complications and symptom management (Figure 4). In the future, patients or for those on HU who have inadequately controlled we will perhaps have the molecular insight to choose medical symptoms. At present, we recommend that all PV and ET cases be assessed for prognosis by risk score (Table 1) and MPN MF symptom burden (MPN-10)21 at the time of diagnosis. In our Management of MF patients requires initial evaluation of the risk opinion, stratifying MPN symptom burden by MPN-10 quartile score using the choice of IPSS, DIPSS, or DIPSS-Plus (when (MPN-10 Q1: 8; MPN-10 Q2: 8-17; MPN-10 Q3: 18-31; MPN-10 karyotype available) and symptom burden (MPN-10). We believe Q4: 32) simplifies symptomatic groupings and allows for objec- that the management of DIPSS low-risk MF patients should be 282 American Society of Hematology Figure 4. Low-risk patients with low symptom burdens for either IFN or new agents; Figure 5). It remains unclear whether (symptom quartiles 1 and 2) may be observed or considered for a patients who are considered low risk by DIPSS but harbor high-risk trial with IFN. Otherwise low-risk patients demonstrating higher Figure 5. Hematology 2014 283 symptom burdens (symptom quartiles 3 and 4) may be considered Dynamic International Prognostic Scoring System for primary myelofi- for ruxolitinib, JAK2 inhibitor trials, or IFN. As per ELN recommendations, intermediate- to high-risk patients, 3. Activating mutation in the regardless of symptomatology, should be urgently assessed for tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. There is mounting evidence to support this approach in 64 polycythemia vera and idiopathic erythrocytosis. For patients not deemed to be allo-SCT 2007;356(5):459-468. Pardanani A, Lasho TL, Finke C, Hanson CA, Tefferi A. Prevalence and opinion that ruxolitinib be initiated or that the patient be considered clinicopathologic correlates of JAK2 exon 12 mutations in JAK2V617F- for enrollment into a JAK2 inhibitor trial. In our experience, using negative polycythemia vera. Martinez-Aviles L, Besses C, Alvarez-Larran A, Cervantes F, Hernandez- dose may lead to anemia or thrombocytopenia in patients with Boluda JC, Bellosillo B. JAK2 exon 12 mutations in polycythemia vera compromised baseline values (ie, hemoglobin 10 g/dL regardless or idiopathic erythrocytosis. Characteristics and dose of 10 mg twice daily for all patients with a hemoglobin 10 clinical correlates of MPL 515W L/K mutation in essential thrombocy- g/dL and platelet counts 100 000 109/L and 5 mg twice daily themia. MPL515 mutations in for platelet counts between 50 and 100 10 /L (ruxolitinib product myeloproliferative and other myeloid disorders: a study of 1182 label). Long-term follow-up of the COMFORT I and II trials do patients. Novel mutations and their functional and clinical relevance in initial 3 months of therapy and that anemia in particular can improve myeloproliferative neoplasms: JAK2, MPL, TET2, ASXL1, CBL, IDH after 6 months. Therefore, it is best to avoid early dose reductions if and IKZF1. If cytopenias become problematic with JAK2 inhibitor myeloproliferative neoplasms with nonmutated JAK2. Somatic mutations of plus a second agent to aid anemia (androgens, immunomodulatory calreticulin in myeloproliferative neoplasms. CALR mutation is a or symptomatic standpoint (including patients with refractory strong independent favorable prognostic variable in primary myelofibro- sis. Paper presented at the European Hematology Association Annual cytopenias) should be considered for trials with other JAK2 Meeting, June 12-15, 2014, Milan, Italy. CALR and ASXL1 or clinical trials using non-JAK2 inhibitors. Type 1 vs type 2 calreticulin The past 10 years after the discovery of the JAK2-V617F mutation mutations in primary myelofibrosis: differences in phenotype and has led to unprecedented advances in our understanding of MPN prognostic impact. JAK2 or CALR mutation status patients has evolved to require a thoughtful and individualized defines subtypes of essential thrombocythemia with substantially differ- approach that incorporates prognosis, disease burden, candidacy of ent clinical course and outcomes. Clinical charecteristics in myeloproliferative neoplasm with calreticulin mutations. JAK inhibitors have made a meaningful impact as single- sented at the European Hematology Association Annual Meeting, June agent therapies for MF and now potentially problematic cases of 12-15, 2014, Milan, Italy. A broad array of ongoing trials will answer the question of 17. Verger E, Dosquet C, Andreoli A, Schlageter M-H, Chomienne C, whether alternative pathways (HDAC inhibitors, telomerase, hedge- Kiladjian J-J. Clinical and molecular response to inerferon alpha therapy hog) alone or in combination with JAK inhibitors are more effective in essential thrombocythemia patients with CALR mutations. Outcome of JAK2/MPL/CALR Conflict-of-interest disclosure: The authors declare no competing triple negative patients with myelofibrosis after allogeneic stem cell financial interests. Paper presented at the European Hematology Associa- tion Annual Meeting, June 12-15, 2014, Milan, Italy. The burden of fatigue and Correspondence quality of life in myeloproliferative disorders (MPDs): an international Ruben A. Mesa, Division of Hematology and Oncology, Mayo Internet-based survey of 1179 MPD patients. Shea Blvd, Scottsdale, AZ 85259; Phone: (480)301- 76. The Myeloproliferative Neoplasm Symptom Assessment Form (MPN-SAF): international pro- References spective validation and reliability trial in 402 patients.
Standard major orthopedic surgery based on the RECORD trials; 2 were in therapy is also preferred among patients in intensive care units with patients undergoing total hip replacement order robaxin 500 mg with visa muscle relaxant adverse effects, with 1 trial having pulmonary emboli and hemodynamic instability or a very extensive different durations of therapy robaxin 500mg cheap spasms in right side of abdomen,22 cheap robaxin 500 mg online spasms from sciatica,23 and 2 were in total knee DVT (eg safe robaxin 500mg spasms left side, phlegmasia cerulea dolens) in whom thrombolytic therapy replacement using 2 different enoxaparin dosing regimens, 40 mg is being considered. Caution should also be exercised in prescribing 12 hours preoperatively and then daily postoperatively or 30 mg rivaroxaban at approved doses to patients at increased risk of BID starting postoperatively. A pooled analysis of the 4 trials, however, indicated reduction of rivaroxaban for creatinine clearance 50 mL/min in a very small increase in bleeding,26 an issue of particular concern to atrial fibrillation, such a dose modification is not recommended for some orthopedic surgeons who will also be reticent in initiating VTE (Table 3). Caution is advised in switching “difficult” to immediate-acting anticoagulants until 12 to 24 hours postopera- manage patients without cancer (ie, recurrent VTE while on tively. In the EINSTEIN studies of patients presenting with acute deep vein Finally, there are practical issues related to the way healthcare thrombosis (DVT) or pulmonary embolism, rivaroxaban alone was systems operate that affect NOAC utilization, particularly for the compared with enoxaparin followed by a vitamin K antagonist for 3, initial treatment of symptomatic DVT entirely in the outpatient 6, or 12 months; the rivaroxaban dose was 15 mg BID for 3 weeks setting. These include the need for preauthorization of NOACs followed by 20 mg daily. The principal safety outcomes of major and departments and among practitioners responsible for follow-up. In the continued-treatment study, rivaroxa- NOACs will replace heparin and vitamin K antagonists as ban reduced the recurrence rate by 82% compared with patients on standard therapy for the majority of patients with an initial placebo, albeit with a small increased risk of major (0. In the AMPLIFY trial, a fixed-dose Antidotes and laboratory monitoring regimen of apixaban alone for 6 months was also shown to be There is no specific antidote available to reverse the anticoagulant noninferior to standard therapy for the treatment of symptomatic effect of dabigatran, but an approach using a monoclonal antibody is VTE, but was associated with significantly less bleeding. Rivaroxaban and apixaban are both highly warfarin); it may reduce the costs of treating VTE by reducing the protein bound and therefore cannot be removed by hemodialysis. A post hoc analysis of pooled data from the results from high drug levels or another etiology (eg, bleeding from 2 trials did not find any difference in outcomes among “fragile” a discrete anatomic site, coagulopathy resulting from liver disease, patients (age 75 years or creatinine clearance 50 mL/min or or disseminated intravascular coagulation) and before some surgical body weight 50 kg), those with active cancer, or those with large procedures (eg, neurosurgery, cardiac surgery). The percentage of cancer patients enrolled in thrombin time have been developed and appear to be sufficiently the trials was also relatively small ( 5%) because oncologists sensitive. However, a normal measurement Adherence, anticoagulation clinics, and medication provides assurance that a significant drug concentration is no longer costs present. Dabigatran prolongs the partial thromboplastin time (PTT), Perhaps the most significant concern with the NOACs is medication but the effects are not dose dependent; although a prolonged PTT adherence. Issues specific to vitamin K antagonists have led to the may indicate the presence of dabigatran, it will not provide an exact development of anticoagulation clinics (so-called Coumadin clin- level of anticoagulant activity. For therapeutic doses of oral factor ics) in many parts of the world, which play a very important role in Xa inhibitors, the prothrombin time (PT) is more sensitive than the optimizing the safety and efficacy of vitamin K antagonists. PTT; however, the results are dependent on the PT reagent used in Warfarin’s long half-life of 40 hours is an advantage for patients the assay and apixaban has less effect on the PT than rivaroxaban. Furthermore, the twice-daily dosing schedules of some provide sensitive and specific assays for measuring drug concentra- NOACs may be more difficult for some patients to adhere to than a tions of oral direct factor Xa inhibitors. The lack of a requirement for recombinant factor VIIa are available for overcoming their antico- regular coagulation monitoring may eliminate the initial and agulant effect in the management of severe bleeding episodes. Healthcare systems are now grappling with how to handle the threatening hemorrhage. If NOACs are prescribed appropriately, the adherence issue with the NOACs. One approach is to have patients current lack of a specific antidote may not be a great drawback in on NOACs be followed by anticoagulation or thrombosis manage- most bleeding situations because there will be relatively few ment clinics. Reports of thrombotic and hemorrhagic complications circumstances in which a reversal agent will be required. This will be an added burden for clinics that are apixaban, respectively, are renally eliminated as active drug. However, with better care coordination and NOAC and this is best done by calculating creatinine clearance bundled payment systems, there should be an impetus for incorporat- using the Cockcroft-Gault formula, which incorporates body weight ing patients on NOACs into these clinics to optimize outcomes (as opposed to other formulas that provide estimates for a body (including reduced need for hospitalizations and emergency depart- surface area of 1. Pharmacists/nurses/physicians with special expertise in All of the trials evaluating dabigatran etexilate or rivaroxaban anticoagulation management can provide the oversight to ensure excluded patients with a creatinine clearance 30 mL/min, whereas that NOACs are prescribed appropriately based upon an individual the ARISTOTLE trial excluded patients with creatinine clearance patient’s history and clinical characteristics. In patients with a creatinine clearance of 30 to 49 Cushman recommended that institutions adopt protocols for NOAC mL/min, the dose of rivaroxaban was reduced to 15 mg, whereas no use in acute VTE that address 6 key components: patient preference, adjustments were made in the RE-LY trial for either the 150 or 110 patient selection, drug interactions, compliance, follow-up, and mg doses of dabigatran etexilate. Based on pharmacokinetic consider- taken into consideration. Even among patients with insurance that ations, a reduced dose of 75 mg BID was approved by the FDA for covers drugs, prescribers should ascertain how much patients pay patients with atrial fibrillation and a creatinine clearance of 15 to 29 out of pocket (copayment) compared with warfarin. High drug costs mL/min in the absence of efficacy and safety data. The FDA also can contribute to reduced medication adherence: patients are known approved rivaroxaban at a dose of 15 mg daily for atrial fibrillation to not fill prescriptions they regard as too expensive or skip/split patients with a creatinine clearance of 15 to 29 mL/min. The FDA also extended the substantial cost for healthcare systems. Many clinicians with The vitamin K antagonists will remain an important anticoagulant expertise in anticoagulant therapy were surprised by these option. They remain the medication of choice for patients with decisions by the FDA, particularly given the current absence of a mechanical heart valves. Therefore, many believe that NOACs have minimal, if any, long-term effects on organ systems and should not be prescribed at any dose in patients with a creatinine physiologic processes other than blood coagulation. JAm mentation Laboratory, Boehringer Ingelheim, Pfizer, Bristol- Coll Cardiol. Meyers Squibb, Janssen Pharmaceuticals, Bayer Healthcare, and 16. Off-label drug use: Dabigatran and apixaban for the botic therapy to prevent stroke in patients who have nonvalvu- prevention and treatment of VTE. Benefit of oral anticoagulant over antiplatelet therapy in atrial fibrillation Correspondence depends on the quality of international normalized ratio control Kenneth A. Bauer, MD, Beth Israel Deaconess Medical Center, 330 achieved by centers and countries as measured by time in Brookline Avenue, Boston, MA 02215; Phone: 617-667-2174; Fax: therapeutic range. Comparison of outcomes among patients randomized to warfarin therapy according to References anticoagulant control: results from SPORTIF III and V. Recent progress in anticoagulant therapy: oral with atrial fibrillation. Kirley K, Qato DM, Kornfield R, Stafford RS, Alexander GC. National trends in oral anticoagulant use in the United States, 3. New oral anticoagulants: which one should versus enoxaparin for thromboprophylaxis after hip arthro- my patient use? Newer oral anticoagulants rivaroxaban versus short-term enoxaparin for the prevention of should be used as first-line agents to prevent thromboembolism venous thromboembolism after total hip arthroplasty: a double- in patients with atrial fibrillation and risk factors for stroke or blind, randomised controlled trial. New oral anticoagulants should not be used as enoxaparin for thromboprophylaxis after total knee arthro- first-line agents to prevent thromboembolism in patients with plasty. Eisert WG, Hauel N, Stangier J, Wienen W, Clemens A, van versus enoxaparin for thromboprophylaxis after total knee Ryn J. Dabigatran: an oral novel potent reversible nonpeptide arthroplasty (RECORD4): a randomised trial. Perzborn E, Roehrig S, Straub A, Kubitza D, Misselwitz F. The prevention of venous thromboembolism after hip or knee discovery and development of rivaroxaban, an oral direct arthroplasty.
It is difficult to judge tension and tie accurately if you do purchase robaxin 500 mg without a prescription spasms in abdomen. This is quite sufficient provided you have taken ‘big bites’ and placed your sutures accurately buy robaxin 500 mg mastercard muscle relaxant gel india. Now do a dye test to check your repair is watertight cheap 500 mg robaxin with mastercard back spasms 8 weeks pregnant. Use 60 ml of dilute methylene blue (or Gentian violet) introduced through a Foley catheter 253 GYNECOLOGY FOR LESS-RESOURCED LOCATIONS (q) (s) (t) (r) (u) Figure 13 (cont) (q) Press over the bladder or ask the patient to cough cheap robaxin 500mg without a prescription spasms of the diaphragm. In the unlikely event of a leak through the suture line put in another suture. The main purpose of a dye test in a simple case is to exclude a second unsuspected fistula, especially an intra-cervical one if the patient has had a cesarean section. The catheter has been sutured to the top of the labia 254 Vesico-vaginal and Recto-vaginal Fistula (a) (e) (b) (f) (c) (g) (h) (d) Figure 14 (a) A large soft mid-vaginal fistula. If possible this should pick up the periosteum of the pubic arch for extra security. Note that the fistula margins fall together because mobilization has been so good. Note the size of the bites 255 GYNECOLOGY FOR LESS-RESOURCED LOCATIONS (a) Figure 15 A large stone has been removed though a suprapubic incision margin between the fistula and the cervix must be clearly seen. Those that extend into the cervical canal can be challenging to close. They should all (b) be approached with caution because the ureters are at risk (Figure 16). The experienced surgeon can find them quickly by eye with a probe, but the best way when starting is to ask the anesthetist to give furosemide 10 mg IV. In 5 min a brisk diuresis will make the orifices obvi- ous if they are near the fistula margin. This can be held by an assistant during the mobiliza- tion and while the first inverting corner suture is inserted as illustrated in the case described below. An easy, type 1 case (c) An example is shown in Figure 17. As a general rule if the fistula is very small (less than 0. However if one can see into the bladder they should always be identified. Use a Babcock’s for- ceps to evert the bladder (Figure 17b,c). Give furosemide 20mg IV (Lasix) if there is still difficulty. Ureteric catheters are notoriously difficult to obtain in Africa and one must rely on outside Figure 16 (a) Diagram illustrating danger area for donations. Beware: probes have been lost up a ureter situ by an assistant, thus protecting it from harm (Figure 18). After repairing a fistula close to the cervix without using ureteric catheters, it is good practice to clamp the urethral catheter and wait to (b) make quite sure that urine is produced. This is to exclude the very small chance that the ureteric orifices have been occluded in the repair. It is essential to realize that any fistula that is below or likely to be close to the ureteric orifices should not be attempted abdominally. An experienced urologist may be able to do it, but it needs full abdominal relaxation, proper retractors, good lighting, a functioning sucker and an ability to (c) catheterize the ureters from inside the bladder. The vault fistula after an emergency hysterectomy for ruptured uterus Most of these are perfectly accessible from the vagina provided they will come down but an abdo- minal repair is an option for the inexperienced vaginal surgeon. Careful bimanual examination will give an indication as to how close the fistula will come to the anterior abdominal wall. If it does then it will be a good case to do from above, pro- vided that of course good lighting, suction and a Figure 17 (a) There is a clear space between this fistula selection of instruments and retractors are available. The external urethral orifice to distal fistula margin is 5 cm. Ideally these should be protected by passing a ureteric The fistula should be visible. Continue the bladder catheter up on each side incision down to the fistula and separate it from the 257 GYNECOLOGY FOR LESS-RESOURCED LOCATIONS nation suggests that the fistula is intra-cervical. Labor long enough to cause death of the baby will usually produce an ischemic injury. The fistula may turn out to be larger and lower than you expect and will be very difficult and unsafe to access from above (unless you are an experienced urologist). Before selecting any patient for an abdominal repair make absolutely certain by dye test and vaginal inspection that the leak is only coming through the cervix or vault not through an occult hole in the vagina (double fistulae do occur). Figure 19 An iatrogenic intra-cervical fistula at the level Prevention at cesarean section of the old lower segment incision is seen. The bladder has been widely opened through the fundus and the interior is In Uganda, two-thirds of patients with fistulae have exposed with a Sim’s speculum. Note the strong traction had their obstructed labor relieved by cesarean sec- applied to the uterus to bring the fistula into view. The remaining one-third ureteric orifices are well below and can easily be demon- have eventually delivered vaginally. If at risk, they are the situation is different; only 15% of fistula patients catheterized have had a cesarean section, because most people live in remote areas far from any hospital. If the fis- ischemic damage may already have occurred by the tula is lower than expected identify the ureteric time of the cesarean section, but the doctor can orifices if necessary with the help of furosemide. The the case has been wisely selected the fistula should lower segment will be very stretched and un- be well above the ureteric openings. Remember that the bladder should be dis- sected well down off the lower segment. The incision in the lower segment should be on the The post-cesarean intra-cervical fistula high side and the lateral ends curved upwards to The regular fistula surgeon will learn to repair minimize inaccessible tears (the left ureter is most at these from below by dissecting up between the risk when repairing a lower segment). There is just one situation When the baby’s head is deeply impacted in the where the repair can be quite easy from above, that pelvis, it is better to get help to push up the head is following an iatrogenic injury without any is- vaginally than to force a hand down between the chemic component. This can be suspected when head and the lower segment. This may produce patient gives the history that she was delivered of a vertical tears and increase the damage already done. The cause of the fistula is almost always birth if possible. Tears in the lower segment can be caused by accidental suture of the bladder into the difficult to suture, and sometimes fistulae are pro- lower uterine segment. In this case repair is quite duced when the doctor inadvertently picks up the possible from above by dissecting between the bladder. This produces an intra-cervical fistula that uterus, cervix and bladder. It should be above the ureteric orifices: if develop fistulae after a cesarean section have a still- there is any doubt give furosemide to identify and birth.
Pantoprazole and rabeprazole were also superior to omeprazole and to lansoprazole for resolution of heartburn (rates 100% for pantoprazole and rabeprazole robaxin 500mg visa muscle relaxant side effects, 87% for omeprazole generic 500mg robaxin spasms after gallbladder surgery, and 82% for lansoprazole) order robaxin 500mg with visa muscle relaxant bruxism. The frequency of adverse events was low (4 patients; 1 robaxin 500mg lowest price quick spasms in lower abdomen. There was 1 small, 12-month, placebo-controlled trial in which pantoprazole 20 mg was effective for maintenance treatment of gastroesophageal reflux disease in patients age 65 or 270 older. An age-based analysis of healing or prevention was not possible in most head-to-head trials, due to the small numbers of older patients. However, 2 trials did assess the impact of age, 5, 106 gender, and race on the incidence of adverse effects. There were no differences between proton pump inhibitors (omeprazole, rabeprazole, esomeprazole) on the basis of these 198 characteristics. The effect of age on eradication rate was also evaluated. This study found higher eradication rates among patients older than 50 years than patients younger than 50, but proton pump inhibitors were not compared. Proton pump inhibitors Page 65 of 121 Final Report Update 5 Drug Effectiveness Review Project In trials comparing a proton pump inhibitor with another drug, the same general statements can be made, but a few findings deserve comment. Studies looking at healing or prevention of nonsteroidal anti-inflammatory drug-induced ulcer included more women than men, with the proportion of women ranging from 62% to 67% and 64% to 83% in the respective types of study. This is most likely due to the greater prevalence among women of diseases requiring long-term nonsteroidal anti-inflammatory drug treatment. Genotype The proton pump inhibitors are all metabolized, largely by the CYP2C19 and CYP3A4 liver enzymes. Theses enzymes are estimated to be deficient in 3% of white and African Americans and 17% to 25% of Asians. The deficiency results in a significantly longer half-life of proton pump inhibitors, although clinically significant accumulation of these drugs has not been shown. While dose adjustments are not required, and adverse effect profiles of the drugs do not differ, 184, 271 there is some evidence that lower doses may be effective in these populations and that 176, 177, 187 rapid metabolizers may have a higher rate of failure to eradicate Helicobacter pylori 272 and to heal esophagitis. Subgroup analysis found no effect by race in 1 study of esomeprazole 4 and lansoprazole in healing of erosive esophagitis. A small study (N=80) found no statistically significant difference at 8 weeks in rate of ulcer healing between rabeprazole 10 mg daily and 108 omeprazole 20 mg daily among patients with differing CYP2C19 genotype. The few adverse events were not analyzed by genotype. A trial of omeprazole in Japanese patients with recurrent 273 esophagitis found no difference in efficacy or safety by genotype. Older patients also metabolize proton pump inhibitors more slowly, resulting in significantly higher drug levels and half-lifes. However, accumulation has not been shown, and dose adjustments are not recommended. One reanalysis of data from 2 trials comparing omeprazole with either ranitidine or cimetidine for reflux esophagitis compared effect in patients 274 age 65 or older with those under age 65. In this analysis there was no difference in healing rate or symptom resolution at 4 or 8 weeks, with a slightly higher proportion of older patients both healed and symptom-free. Withdrawals due to adverse event were higher in the older group, 7. Similar data are not available for other proton pump inhibitors. Comorbidity In an uncontrolled, non-randomized open-label study, patients with peptic ulcer and comorbid 275 liver disease were given 6 to 8 weeks of rabeprazole 10 mg to 20 mg. Eleven of 108 patients (10%) reported 21 adverse drug events, resulting in 5 withdrawals (5%) and an additional 5 patients with an adverse event were lost to follow up. Two patients (2%) had adverse events that were rated as serious, 1 had an elevated bilirubin level, and the other had hepatic encephalopathy. Concomitant medications Two good quality observational studies assessed the impact of a potential drug interaction between proton pump inhibitors and clopidogrel following an acute coronary syndrome (ACS). A cohort study of 8205 patients who were discharged after an ACS and were prescribed clopidogrel between October 2003 and January 2006 were examined to Proton pump inhibitors Page 66 of 121 Final Report Update 5 Drug Effectiveness Review Project determine if the rate of death or rehospitalization for ACS was affected by concomitant use of a 276 proton pump inhibitor. Of these patients, 64% were prescribed a proton pump inhibitor. Multivariable analysis found that there was an increased risk of death or rehospitalization for ACS in those patients taking both clopidogrel and a proton pump inhibitor; adjusted odds ratio 1. The analysis controlled for multiple variables, included demographic characteristics, comorbidities, previous cardiac history, and other medications. In patients who had period with and without a proton pump inhibitor, but continued clopidogrel use, the risk of the primary outcome was also increased during the proton pump inhibitor periods; adjusted odds ratio 1. In addition to the primary outcome, secondary outcomes were evaluated. The risk of rehospitalization for ACS was increased (adjusted odds ratio 1. The authors also conducted a nested case-control analysis with these data in an attempt to confirm their findings, resulting in an adjusted odds ratio of 1. Multiple sensitivity analyses were conducted, with no meaningful change to the results. This study was conducted using data from the Veteran’s Affairs hospitals, and no patients were taking esomeprazole. Too few patients were taking pantoprazole or lansoprazole to be able to conduct individual analyses, but omeprazole and rabeprazole resulted in increased adjusted odds ratios for the primary outcome (adjusted odds ratios: 1. Analysis by dose of proton pump inhibitor indicated did not indicate a dose-response relationship. A population-based nested case-control study examined data from all patients in Ontario, Canada who were prescribed clopidogrel after hospital discharge following a myocardial 277 infarction between April 2002 and December 2007. Among this group, cases were identified as patients who were rehospitalized for myocardial infarction within 90 days of discharge (N=734), while controls were those who were not. Controls were identified in a 3:1 ratio to cases and matched on age, percutaneous coronary intervention, and a validated risk score (N=2057). Proton pump inhibitor exposure was defined as current (within 30 days of rehospitalization), previous (31 to 90 days) or remote (91 to 180 days). The logistic regression analysis controlled for demographic variables, socioeconomic status, Charlson comorbidity index, length of stay during initial admission for myocardial infarction, and 9 comorbid conditions (for example diabetes). A similar adjusted odds ratio was found in this study as the cohort study; 1. Previous or remote use was not associated with an increased risk of recurrent myocardial infarction. All-cause mortality was again not affected statistically significantly (adjusted odds ratio 0. Analysis of recurrent myocardial infarction within 1 year of initial discharge also indicated an increased risk with current proton pump inhibitor use; odds ratio 1. Because pantoprazole does not inhibit the P450 2C19 enzyme system responsible for activation of clopidogrel, it has been suggested that it may not result in a clinically-relevant drug interaction. An analysis of pantoprazole alone (N=cases 46, controls 125) found no statistically significant increase in risk (adjusted odds ratio 1.
Robaxin 500 mg amex. All alpha agonists (alpha1&2).