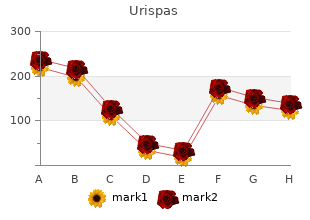
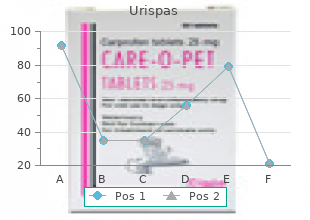
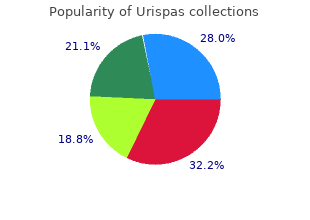
Georgetown University. B. Giacomo, MD: "Order online Urispas no RX - Safe online Urispas".
In residential centres where children self administer medicines cheap urispas 200mg free shipping spasms in 7 month old, a risk assessment should be carried out and recorded in the care plan generic urispas 200 mg amex spasms spanish. It should determine: that the resident is able to look after and self administer their own medicines whether any monitoring is needed to assess the ability to self-administer or willingness to take the medicines as prescribed that medicine has been taken as prescribed (either by seeing this directly or by asking the resident) who has recorded that the medicine has been taken urispas 200mg with visa muscle relaxant for headache. Residential services should ensure that their process for self‑administration of Schedule 2 and 3 controlled drugs includes additional specific information about: obtaining or ordering Schedule 2 and 3 controlled drugs storing Schedule 2 and 3 controlled drugs recording supply of Schedule 2 and 3 controlled drugs to residents disposal of unused or expired Schedule 2 and 3 controlled drugs generic 200mg urispas overnight delivery spasms medication. Residents should be offered the medicines at the times they are experiencing the symptoms either by telling a member of staff or by staff identifying the resident’s need as outlined in the care plan. Staff who may need to administer such medicine require additional training so that they can administer it safely and confidently in an emergency. If a second dose of medicine is prescribed, then the prescription must state the period of time after administration of the first dose in which the second dose can be administered. Medicines used for the management of seizures should be reviewed and evaluated on a regular basis. The centre’s medicines management policy should include guidance to staff on how to manage refusal of medicines. This guidance should include the actions to be taken if medicines are refused, who to contact and the documentation to be completed. If a resident agrees to take a medicine later than the prescribed time, this must be documented clearly in the medicines administration record. If a medicine is given at a later time than prescribed, the prescriber should be contacted to ensure that there are no contra-indications. If there is a pattern where a resident often refuses medicine, a plan must be put in place with involvement of the staff, multidisciplinary team, the resident and their representatives, if appropriate. This plan must be reviewed on a regular basis, in line with the relevant legislation or more often if circumstances change. There are legal requirements for the storage, administration, records and disposal of Schedule 2 and 3 controlled drugs. All medicines, including Schedule 2 and 3 controlled drugs (except those for self administration) are administered by a registered nurse or medical practitioner in older persons’ residential services. In social care settings such as residential services for people with disabilities, other personnel may be trained to administer medicines. In order to administer a Schedule 2 and 3 controlled drug, all the steps involved in giving any other medicine should be followed. The receipt, administration, management and disposal of controlled drugs are recorded in accordance with relevant legislative requirements, national guidelines and professional guidelines; for example, An Bord Altranais agus Cnáimhseachais na hÉireann guidelines. Schedule 2 and 3 controlled drugs (including those for self-administration) must be secured in a manner that meets legislative requirements as set out by the Misuse of Drugs Regulations. They should be locked in a separate cupboard or container from other medicinal products to ensure further security. Policies and procedures should be in place for the checking of stock balance for each transaction of controlled drugs. A record of the receipt, administration and disposal of Schedule 2 controlled drugs is required to be maintained in a bound controlled drugs register. As per guidance issued 27 Medicines Management Guidance Health Information and Quality Authority by An Bord Altranais agus Cnáimhseachais na hÉireann, a count for controlled drugs should be carried out at all staff changeover shifts. All these terms refer to medicines that can be bought without a prescription and are used to treat minor ailments. They are safe and effective when the directions on the label are followed and are taken as directed by the healthcare professional. It is important that information and advice is sought from an appropriate healthcare professional (pharmacist, nurse, or doctor) or product information (summary of product characteristics or patient information leaflet) before the administration of these medicines. The healthcare professional should be made aware of the medicines the resident is prescribed. It includes names of medicines, dosage, frequency and route, in order to identify any discrepancies and to ensure any changes are documented and communicated. This reconciliation is done to avoid medication incidents such as omissions, duplications, incorrect dosing, or drug interactions. Medication reconciliation aims to provide residents and healthcare professionals with the correct medicines at all transitions in care, within and between health and social care services. Transitions in care include changes in setting, service, practitioner, or level of care. Medication reconciliation is considered complete when each medicine that a person is taking has been actively continued, discontinued, held or modified at each point of transfer, and these details have been communicated to the next care provider. A medicines review should be a structured and collaborative healthcare service provided to residents in residential services. Good practice suggests the review of medicines should involve the resident, his or her representative as appropriate, prescriber, pharmacist, nursing staff and other relevant members of the health and social care team. The medicines review should take place in line with the relevant legislation or more frequently where there is a significant change in the resident’s care, medicines or condition. Comprehensive information about the resident and their medicine use should be collated and assessed in order to identify and meet medicine related needs and to identify, resolve and prevent medicine related problems. This enhances the resident’s quality of life and optimises the benefits achieved from medicine use. The medicines review should review all prescribed, over-the-counter and complementary medicines used by the resident. The resident’s medicines adherence, side-effects, adverse drug events and monitoring test results form part of the review. Particular attention should be given to the following: antipsychotic medicines sedative medicines medicines for the management of depression antiepileptic medicines analgesia or pain medicines laxatives and treatments for constipation anticoagulant and antiplatelet medicines antimicrobial medicines 30 Medicines Management Guidance Health Information and Quality Authority diuretic medicines influenza and pneumococcal vaccines non-steroidal anti-inflammatory drugs medicines and their potential interactions including any drug-nutrient interactions appropriate polypharmacy and problematic polypharmacy. The medicines review should be documented in the resident’s medical notes detailing changes that have been made or that no changes have been made. Prescription and administration records should be updated following such reviews to reflect any changes that have been made. All relevant changes to the resident’s medicines following the review are clearly documented and a note is also made if no changes are to be made. Other methods of disposal include returning them to the supplier; for example, a community pharmacy. The supplier can then ensure that medicines are disposed of in accordance with current waste regulations. The situations when medicines might need to be disposed of include: A resident’s treatment is changed or discontinued — the remaining supplies of it should be disposed of safely with the person’s consent. When applicable, this is stated in the product information leaflet which accompanies the medicine. When medicines are disposed of a record should be made to show that they were handled properly. The following information should be recorded: date of disposal or date of return to pharmacy name and strength of medicine quantity removed resident for whom medicines were prescribed or purchased signature of the member of staff who arranges disposal of the medicines. If medicines are disposed of within the service, clear policies and procedures should be in place. Disposal of waste medicines must be in compliance with waste management legislation.
Common clindamycin toxicities include fever discount urispas 200 mg line kidney spasms causes, rash buy urispas 200mg lowest price muscle relaxant vitamins minerals, nausea urispas 200 mg without prescription spasms versus spasticity, diarrhea (including pseudomembranous colitis or diarrhea related to Clostridium difficile toxin) cheap 200 mg urispas fast delivery muscle relaxant list by strength, and hepatotoxicity. Common atovaquone toxicities include nausea, vomiting, diarrhea, rash, headache, hepatotoxicity, and fever. Drug interactions between anticonvulsants and antiretroviral agents should be evaluated carefully; if necessary, doses should be adjusted or alternative anticonvulsants should be used. In patients who adhere to their regimens, disease recurrence is unusual in the setting of chronic maintenance therapy after an initial clinical and radiographic response. Although sulfadiazine is routinely dosed as a four-times-a-day regimen, a pharmacokinetic study suggests bioequivalence for the same total daily dose when given either twice or four times a day,69 and limited clinical experience suggests that twice-daily dosing is effective. Toxoplasmosis diagnostic considerations are the same in pregnant women as in non-pregnant women. With respect to congential toxoplasmosis, the risk of transmission is highest in the setting of an acute maternal infection as compared to reactivation. While the risk of transmission increases with advancing gestational age, the severity of fetal sequelae is more pronounced the earlier in gestation the fetus is affected. The value of routine toxoplasmosis screening programs is debated in the United States but generally accepted in other countries. In countries such as France where pregnant women are universally screened and treated, infected offspring are reported to have primarily mild disease and rarely severe disease. Studies published since 2007 support treatment of toxoplasmosis during pregnancy in an effort to decrease vertical transmission and reduce the severity of clinical signs in the offspring. Spiramcyn is not teratogenic, does not treat infection in the fetus and is primarily indicated for fetal prophylaxis. Pyrimethamine should not be used in the first trimester because of teratogenicity concerns. The infant’s care provider should be notified of maternal sulfa use in late pregnancy. While there are limited data on atovaquone safety in humans, preclinical studies have not demonstrated toxicity. Maintenance therapy should be provided, using the same indications as for non-pregnant women. Outbreak of central-nervous-system toxoplasmosis in western Europe and North America. Central-nervous-system toxoplasmosis in homosexual men and parenteral drug abusers. Use of a clinical laboratory database to estimate Toxoplasma seroprevalence among human immunodeficiency virus-infected patients. Toxoplasma gondii infection in the United States, 1999 2004, decline from the prior decade. Incidence and risk factors for toxoplasmic encephalitis in human immunodeficiency virus-infected patients before and during the highly active antiretroviral therapy era. Pyrimethamine for primary prophylaxis of toxoplasmic encephalitis in patients with human immunodeficiency virus infection: a double-blind, randomized trial. Risk factors for Toxoplasma gondii infection in mothers of infants with congenital toxoplasmosis: Implications for prenatal management and screening. Use of the peroxidase-antiperoxidase method to demonstrate toxoplasma in formalin fixed, paraffin embedded tissue sections. A randomized trial of three antipneumocystis agents in patients with advanced human immunodeficiency virus infection. A randomized trial comparing pyrimethamine plus clindamycin to pyrimethamine plus sulfadiazine. Treatment of central nervous system toxoplasmosis with pyrimethamine/ sulfadiazine combination in 35 patients with the acquired immunodeficiency syndrome. Folinic acid supplements to pyrimethamine-sulfadiazine for Toxoplasma encephalitis are associated with better outcome. Cotrimoxazole for treatment of cerebral toxoplasmosis: an observational cohort study during 1994-2006. Clarithromycin-minocycline combination as salvage therapy for toxoplasmosis in patients infected with human immunodeficiency virus. The immune inflammatory reconstitution syndrome and central nervous system toxoplasmosis. Plasma pharmacokinetics of sulfadiazine administered twice daily versus four times daily are similar in human immunodeficiency virus-infected patients. Maintenance therapy with cotrimoxazole for toxoplasmic encephalitis in the era of highly active antiretroviral therapy. Low incidence of congenital toxoplasmosis in children born to women infected with human immunodeficiency virus. Congenital toxoplasmosis occurring in infants perinatally infected with human immunodeficiency virus 1. Mother-to-child transmission of toxoplasmosis: risk estimates for clinical counselling. Performance of Polymerase Chain Reaction Analysis of the Amniotic Fluid of Pregnant Women for Diagnosis of Congenital Toxoplasmosis: A Systematic Review and Meta- Analysis. Prenatal diagnosis using polymerase chain reaction on amniotic fluid for congenital toxoplasmosis. Congenital Toxoplasmosis in France and the United States: One Parasite, Two Diverging Approaches. Prenatal treatment for serious neurological sequelae of congenital toxoplasmosis: an observational prospective cohort study. Efficacy of rapid treatment initiation following primary Toxoplasma gondii infection during pregnancy. Risk factors for retinochoroiditis during the first 2 years of life in infants with treated congenital toxoplasmosis. Toxoplasmosis in the fetus and newborn: an update on prevalence, diagnosis and treatment. Safety and toxicity of sulfadoxine/pyrimethamine: implications for malaria prevention in pregnancy using intermittent preventive treatment. Sulfadiazine rheumatic fever prophylaxis during pregnancy: does it increase the risk of kernicterus in the newborn? A difference in mortality rate and incidence of kernicterus among premature infants allotted to two prophylactic antibacterial regimens. Cryptosporidium can also infect other gastrointestinal and extraintestinal sites, especially in individuals whose immune systems are suppressed. The three species that most commonly infect humans are Cryptosporidium hominis, Cryptosporidium parvum, and Cryptosporidium meleagridis. Viable oocysts in feces can be transmitted directly through contact with infected humans or animals, particularly those with diarrhea. Oocysts can contaminate recreational water sources such as swimming pools and lakes, and public water supplies and may persist despite standard chlorination (see Appendix: Food and Water-Related Exposures). Person-to-person transmission is common, especially among sexually active men who have sex with men.
A randomised controlled trial has demonstrated definitive efficacy over no change in treatment discount urispas 200 mg online muscle relaxant sciatica. More relaxed forms of the diet have raised the possibility of it being available to use over a wide age range buy urispas 200 mg with amex muscle relaxant list by strength. Intravenous immunoglobulins have been used with varying (usually very limited) discount urispas 200 mg otc muscle relaxant names, success in buy urispas 200mg on-line infantile spasms 2 year old,52,53 intractable epilepsies including children with both the West and Lennox-Gastaut 54,55 syndromes. There are marked variations in the frequency of courses, duration of treatment and doses of this particular therapy and there is as yet no established or universally accepted mechanism of action. Drug choice in childhood epilepsy should, wherever possible, be evidence based as in older individuals. However, there are few randomised controlled trials on which to base drug choice within the epilepsy syndromes. This in part reflects the logistical and ethical difficulties as well as the expense in conducting paediatric trials. Nevertheless, the principal should still be to try and base treatment strategies on robust evidence. They state that focal epilepsies in children older than four years of age have a similar clinical expression to focal epilepsies in adolescents and adults. In refractory focal epilepsies, the results of efficacy trials performed in adults could to some extent be extrapolated to children, provided the appropriate dose and safety data are established. For syndromes limited to childhood, sufficient experience needs to be gained in this 56 population before a new medicinal product may be registered for these indications in children ; predictably such experience is likely to be largely anecdotal unless data can be obtained from well-conducted national or international randomised controlled trials. Many studies are conducted on the basis of seizure type rather than syndrome, are limited in duration and reveal little in the way of long-term effects. Further, a recent randomised double-blind trial in the treatment of childhood absence epilepsy comparing ethosuxuimide, sodium valproate and lamotrigine showed superior efficacy of sodium valproate and 59 ethosuximide over lamotrigine, but some neuropsychological advantage to ethosuximide. There has been increasing concern about the effect of sodium valproate on the unborn child of mothers taking the medication – both an increased risk of malformations, as well as cognitive delay in later childhood. For this reason the medication is not recommended as first line in girls of child-bearing age, and when considered, the risks of taking the medication need to be weighed against the risk of the epilepsy itself in each individual. Epilepsies associated with focal seizures are slightly less common in children in contrast to adults and for these individuals carbamazepine is the usual preferred treatment. Vigabatrin is particularly effective in 12 treating infantile spasms caused by tuberous sclerosis but appears to be slightly less effective 61,62 than tetracosactide or prednisolone in treating spasms due to other aetiologies. However there are currently differences of opinion regarding the treatment of infantile spasms, in part reflecting clinicians’ concerns over drug safety and in part availability of medication. Which is used will depend on family and physician choice, weighing up the risk:benefit of the treatment involved. Although use of vigabatrin in adults and older children has been associated with 21 visual field constriction, this appears to be related to dose and duration of treatment and does not necessarily prevent or reduce the use of this drug in treating infantile spasms when weighed up against the risk of short-term high-dose steroids. In Dravet syndrome, previously called severe myoclonic epilepsy of infancy, medications of choice are sodium valproate, clobazam and topiramate. Furthermore a well-constructed randomised crossover study demonstrated stiripentol, a cytochrome P450 inhibitor, to be 63 significantly more effective than placebo when added to sodium valproate and clobazam ; however, this drug may be associated with significant somnolence as well as loss of appetite. Several studies have been conducted evaluating treatments against placebo in Lennox-Gastaut syndrome as add-on therapy. Overall the authors concluded that no study to date had shown any one drug to be effective over and above another but lamotrigine, rufinamide, clobazam, topiramate and felbamate may be helpful as add-on 66 therapy. Therefore until further research has been undertaken clinicians will need to continue to consider each patient individually, taking into account the potential benefit of each therapy weighed against the risk of adverse effects. These must be effective (preferably with a broad spectrum of action against a wide range of seizure types), safe and be available in child-friendly formulation. In this regard, it is common for a child to be falsely described as being refractory to treatment because they have been prescribed the wrong drug for their epilepsy syndrome. The classic example is the use of carbamazepine or oxcarbazepine for juvenile-onset absence or juvenile myoclonic epilepsy, when it is known to exacerbate both the myoclonic and absence seizures which characterise these syndromes. Consequently the prescribing mantra must be ‘if I add, what can I take away’ to avoid dangerous polypharmacy. In individual cases of torsades de pointes there are often multiple risk factors present. The 8,9,10,11 main risk factors which should be considered are: Potentially Modifiable A list of medicines Electrolyte Disturbances (in particular hypokalaemia, hypomagnesaemia and more known to prolong the rarely hypocalcaemia). It is recommended that you check the lists for drugs commonly used in your area of practice to familiarise yourself with the risks. Antimicrobials Antipsychotics (all have some risk) Erythromycin Risperidone Clarithromycin Fluphenazine Moxifloxacin Haloperidol Fluconazole Pimozide Ketoconazole Chlorpromazine Antiarrhythmics Quetiapine Dronedarone Clozapine Sotalol Antidepressants Quinidine Citalopram/escitalopram Amiodarone Amitriptyline Flecainide Clomipramine Dosulepin Others Doxepin Methadone Imipramine Protein kinase inhibitors e. The risk of torsades de pointes depends on patient factors and medication history. The decision should be made on a case by case basis taking into account any additional risk factors the patient has. Domperidone: small risk of serious ventricular arrhythmia and sudden cardiac death. Changes to the contents are published in Hormone Preparations – Systemic 90 monthly updates. Alternatively there is a nominal charge for an annual subscription Respiratory System & Allergies 211 to the printed Schedule publications. To Sensory Organs 219 access either of these subscriptions visit our subscription website www. This includes community pharmaceuticals, hospital pharmaceuticals, vaccines and increasingly, hospital medical devices. The processes we generally use are outlined in our Operating Policies and Procedures. This medicine is an unapproved medication supplied under Section 29 of the Medicines Act 1981. Community Pharmaceutical costs met by the Government Most of the cost of a subsidised prescription for a Community Pharmaceutical is met by the Government through the Combined Pharmaceutical Budget. The Government pays a subsidy for the Community Pharmaceutical to pharmacies, and a fee covering distribution and pharmacy dispensing services. The subsidy paid to pharmacies does not necessarily represent the final cost to Government of subsidising a particular Community Pharmaceutical. Patient costs Everyone who is eligible for publicly funded health and disability services should in most circumstances pay only a $5 co-payment for subsidised medicines, although co-payments can vary from $0 to $15. A patient may also pay additional fees for services such as after-hours dispensing and special packaging. For more information on patient co-payments or eligibility please visit http://www. Subsidy Once approved, the applicant will be provided a Special Authority number which must appear on the prescription. The authority number can provide access to subsidy, increased subsidy, or waive certain restrictions otherwise present on the Community Pharmaceutical. Some approvals are dependent on the availability of funding from the Combined Pharmaceutical Budget. For some Special Authority Community Pharmaceuticals, not all indications that have been approved by Medsafe are subsidised.
There is today much opportunity to expand access to cancer care with existing low-cost products buy urispas 200mg with mastercard muscle relaxant potency. Twenty percent of breast cancer patients require trastuzumab (Herceptin) that is prohibitively expensive today buy discount urispas 200 mg online muscle relaxant remedies. Eighty percent of breast cancer cases can be treated with older discount urispas 200mg without prescription spasms right buttock, less costly medicines generic urispas 200 mg free shipping muscle relaxant used by anesthesiologist. It is essential that governments take action to ensure the price of trastuzumab comes down. Advocating for affordable trastuzumab will be more effective in an environment where breast cancer treatment and care is available to all women. It will be an opportunity to include proven effective treatments (regardless of cost) and provide a basis for further action to ensure availability and affordability of these essential cancer medicines. Once cancer medications are included in the core list, such a list can form the basis for inclusion in the World Health Organization’s Prequalification Program’s Expression of Interest, help attract low-cost quality generic suppliers and guide countries’ selection. An overview of price ranges by the Global Task (see Table 3) shows wide ranges in prices paid for cancer medications in low- and middle- income countries. Publicly available drug price and source information should be made available and regularly updated. In the cases where generic manufacturing is not possible because of a patent, licenses should be made available. Patent holders should be incentivized to license their patents of essential cancer drugs to generic manufacturers. The Medicines Patent Pool can provide a model for health-oriented licensing and licensing terms. Licenses with a large geographical scope help to create economies of scale and thus lower the cost of production. Governments should provide compulsory licenses to generic producers in the case a patent holder refuses to license on reasonable terms. It will be important to protect the flexibilities in intellectual property law that countries have to remedy the negative effect of drug patents. The use of these flexibilities to increase access to cancer drugs is completely legal under international law. Countries have to intervene when patents cause access problems and patent holders refuse to provide licenses to the patents. This may require agreements at international level on reference pricing to prevent high-income countries demanding discount levels intended for low- and middle-income countries. A very effective mechanism for differential pricing of patented medicines is through licensing. Production of lower-priced products by generic companies offers the steepest discounts. Because products produced under a license are marketed under a different brand, there is no risk of flow back to high-income markets, which has always been a concern of originator companies in implementing differential pricing. Demands for cancer treatment in low- and middle-income countries will increase and a response by health authorities in many countries is long overdue. This lack of response cannot be explained by the high cost of cancer medicines only. Many of the products used in cancer treatment are available from multiple sources at affordable price levels. To make those medicines available to cancer patients, governments should put in place, and sustain, cancer screening and treatment strategies. Those medicines are often very highly priced and out of reach of people and health systems in low- and middle-income countries. Essential cancer medicines whether old or new, should be made available in the context of cancer care. This will require action by governments and companies to ensure these treatments are affordable. In case of single-source cancer drug supply, relying on differential pricing alone does not provide the sustained decrease in price that is necessary. Where patents are barriers to access generic cancer medication, companies should offer licenses and if they fail to do so governments should use compulsory licensing strategies. However, for all of this to happen we need a vocal civil society that demands drastic change in the current situation. Grady (2009) ‘How much is life worth: cetuximab, non-small cell lung cancer, and the $440 billion question’, J Natl Cancer Inst. N (2009) ‘Limits on Medicare’s Ability to Control Rising Spending on Cancer Drugs’ Engl J Med 360: 626–633doi: 10. Wittes (2012) ‘In cancer care, cost matters’ New York Times 15 October: pA25 (http://www. Jackson (2013) ‘Gilead critic sponsors voter initiative to limit drug pricing in San Francisco’. Ford (2014) ‘Minimum costs for producing hepatitis C direct-acting antivirals for use in large-scale treatment access programs in developing countries’, Clin Infect Dis. Kumar (2005) ‘Change in the age structure of India’s population (1881-2001)’, Dialogue 6: 445–457. Nandkumar (2010) ‘Projection of number of cancer cases in India (2010- 2020) by cancer group’, Asian Pacific Journal of Cancer Prevention 11: 1045–1049. Swaminathan (2005) ‘Cancer: current scenarios, intervention strategies and projections for 2015’, National Committee on Macroeconomics and Health Background Papers: Burden of disease in India, New Delhi 52 Ibid. Mithral (1994) ‘Breast cancer screening: the case for physical examination without mammography’, Lancet, 343: 342–344. Fox (2009) Global strategies to reduce the price of antiretroviral medicines: evidence from transactional databases, http://www. Health Action International is currently carrying out a project to map external reference pricing practices for medicines with the support of Dfid. Ixabepilone is indicated as monotherapy for the treatment of metastatic or locally advanced breast cancer in patients whose tumors are resistant or refractory to anthracyclines, taxanes, and capecitabine. Research reports This research report was commissioned by Oxfam and written to share research results, to contribute to public debate and to invite feedback on development and humanitarian policy and practice. The copyright holder requests that all such use be registered with them for impact assessment purposes. For copying in any other circumstances, or for re-use in other publications, or for translation or adaptation, permission must be secured and a fee may be charged. Japan: The Advertising Department, Subscribers may reproduce tables of con- Subscription prices are available upon Elsevier K. Subscriptions are European Journal Commercial Sales, compilations and translations. Priority fax: (+44) (0) 20 7424 4433; store or use electronically any material rates are available upon request.
Order 200 mg urispas overnight delivery. Протеины и анаболические стероиды в чем между ними разница и что вреднее.