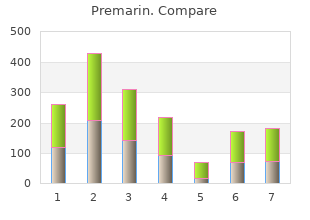
Tougaloo College. J. Tyler, MD: "Purchase online Premarin - Cheap online Premarin no RX".
Patient selection for diagnostic coronary angiography and hospital-level percutaneous coronary intervention appropriateness: insights from the National Cardiovascular Data Registry order 0.625 mg premarin otc women's health center naperville il. Use of diagnostic coronary angiography in women and men presenting with acute myocardial infarction: a matched cohort study generic premarin 0.625 mg mastercard women's health center new prague mn. Iatrogenic left main coronary artery dissection: incidence buy discount premarin 0.625mg menopause 2 years got period, classification 0.625mg premarin free shipping women's health recipe finder, management, and long-term follow-up. Ventricular arrhythmia onset during diagnostic coronary angiography with a 5F or 4F universal catheter. Stroke in patients undergoing coronary angiography and percutaneous coronary intervention: incidence, predictors, outcome and therapeutic options. Safety of coronary angiography and percutaneous coronary intervention via the radial versus femoral route in patients on uninterrupted oral anticoagulation with warfarin. Associations between acute kidney injury and cardiovascular and renal outcomes after coronary angiography. Acute kidney injury following coronary angiography is associated with a long-term decline in kidney function. Nephrotoxicity of ionic and nonionic contrast media in 1196 patients: a randomized trial. Intravenous N-acetylcysteine for prevention of contrast-induced nephropathy: a meta-analysis of randomized, controlled trials. Sodium bicarbonate for the prevention of contrast induced- acute kidney injury: a systematic review and meta-analysis. Systematic review: sodium bicarbonate treatment regimens for the prevention of contrast-induced nephropathy. Optimizing radiation safety in the cardiac catheterization laboratory: a practical approach. Effect of a real-time radiation monitoring device on operator radiation exposure during cardiac catheterization: the radiation reduction during cardiac catheterization using real-time monitoring study. Radial versus femoral access in patients with acute coronary syndromes undergoing invasive management: a randomised multicentre trial. Radial artery spasm during transradial cardiac catheterization and percutaneous coronary intervention: incidence, predisposing factors, prevention, and management. A comparison of the use of traditional hand injection versus automated contrast injectors during cardiac catheterization. Meta-analysis of the effect of automated contrast injection devices versus manual injection and contrast volume on risk of contrast-induced nephropathy. Frequency, outcome, and appropriateness of treatment of nonionic iodinated contrast media reactions. Congenital anomalous aortic origins of the coronary arteries in adults: a Tunisian coronary arteriography study. Repair of anomalous origin of the left coronary artery from the pulmonary artery in infants and children. Anomalous origin of the right coronary artery from the left anterior descending artery: review of the literature. Coronary arteriovenous fistulas in the adults: natural history and management strategies. Comparison of clinical interpretation with visual assessment and quantitative coronary angiography in patients undergoing percutaneous coronary intervention in contemporary practice: the Assessing Angiography (A2) project. High platelet reactivity on clopidogrel therapy correlates with increased coronary atherosclerosis and calcification: a volumetric intravascular ultrasound study. Continuum of vasodilator stress from rest to contrast medium to adenosine hyperemia for fractional flow reserve assessment. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. Fractional flow reserve-guided versus angiography- guided coronary artery bypass graft surgery. Incidence and predictors of coronary stent thrombosis: evidence from an international collaborative meta-analysis including 30 studies, 221,066 patients, and 4276 thromboses. Assessment of fibrous cap thickness by optical coherence tomography in vivo: reproducibility and standardization. Intravascular ultrasound: principles, image interpretation, and clinical applications. Definitions and methodology for the grayscale and radiofrequency intravascular ultrasound and coronary angiographic analyses. Tissue characterisation using intravascular radiofrequency data analysis: recommendations for acquisition, analysis, interpretation and reporting. Spontaneous coronary artery dissection: prevalence of predisposing conditions including fibromuscular dysplasia in a tertiary center cohort. Impact of intravascular ultrasound imaging on early and late clinical outcomes following percutaneous coronary intervention with drug-eluting stents. Intravascular ultrasound-guided implantation of drug-eluting stents to improve outcome: a meta-analysis. Expert consensus statement on the use of fractional flow reserve, intravascular ultrasound, and optical coherence tomography: a consensus statement of the Society of Cardiovascular Angiography and Interventions. Imaging of vulnerable plaques using near-infrared spectroscopy for risk stratification of atherosclerosis. Morphometric assessment of coronary stenosis relevance with optical coherence tomography: a comparison with fractional flow reserve and intravascular ultrasound. Pathophysiology of acute coronary syndrome assessed by optical coherence tomography. Assessment of culprit lesion morphology in acute myocardial infarction: ability of optical coherence tomography compared with intravascular ultrasound and coronary angioscopy. Incidence and clinical significance of poststent optical coherence tomography findings: one-year follow-up study from a multicenter registry. Delayed coverage in malapposed and side-branch struts with respect to well-apposed struts in drug-eluting stents: in vivo assessment with optical coherence tomography. Examination of the in vivo mechanisms of late drug- eluting stent thrombosis: findings from optical coherence tomography and intravascular ultrasound imaging. Incidence, predictors, morphological characteristics, and clinical outcomes of stent edge dissections detected by optical coherence tomography. Heart disease and stroke statistics—2013 update: a report from the American Heart Association. Differences in the incidence of congestive heart failure by ethnicity: the Multi-Ethnic Study of Atherosclerosis. Use of both systems in conjunction provides a reasonable framework for clinician communication and patient prognostication. Following thorough history and physical examination together with initial diagnostic testing, imaging (e. Further, the history helps to evaluate incongruent results that may emerge during the diagnostic process, and it can obviate the need for needless further testing. None of these is entirely sensitive or specific for identifying the presence of severe congestion (Table 21. Probing more deeply into the current level of activity may uncover a decline in exercise capacity that is not immediately apparent.
Lightheadedness/presyncope/syncope when there is very low clinical suspicion for cardiovascular disease I (3) 9 buy 0.625 mg premarin overnight delivery women's health center lebanon nh. Syncope when no other symptoms or signs of cardiovascular disease are present A (7) Evaluation of Ventricular Function 10 cheap premarin 0.625 mg with mastercard women's health issues in sri lanka. Evaluation of left ventricular function with previous ventricular function evaluation showing normal function (e purchase premarin 0.625 mg without a prescription breast cancer football socks. Routine perioperative evaluation of ventricular function with no symptoms or signs of cardiovascular disease I (2) 14 order 0.625mg premarin with visa women's health clinic kalgoorlie. Routine perioperative evaluation of cardiac structure and function before noncardiac solid-organ transplantation U (6) Pulmonary Hypertension 15. Evaluation of suspected pulmonary hypertension, including evaluation of right ventricular function and estimated pulmonary artery pressure A (9) 16. Routine (<1 year) reevaluation of known pulmonary hypertension without change in clinical status or findings on cardiac examination I (3) 17. Routine (≥1 year) reevaluation of known pulmonary hypertension without change in clinical status or findings on cardiac examination A (7) 18. Hypotension or hemodynamic instability of uncertain or suspected cardiac cause A (9) 20. Assessment/monitoring of volume status in a critically ill patient U (5) Myocardial Ischemia/Infarction 21. Evaluation of a patient without chest pain but with other features of an ischemic equivalent or laboratory markers indicative of ongoing myocardial A (8) infarction 23. Suspected complication of myocardial ischemia/infarction, including but not limited to acute mitral regurgitation, ventricular septal defect, free wall A (9) rupture/tamponade, shock, right ventricular involvement, heart failure, or thrombus Evaluation of Ventricular Function After Acute Coronary Syndrome 24. Respiratory failure or hypoxemia when a noncardiac cause of respiratory failure has been established U (5) Pulmonary Embolism 28. Routine reevaluation of previous pulmonary embolism with normal right ventricular function and pulmonary artery systolic pressure I (1) 31. Reevaluation of known pulmonary embolism after thrombolysis or thrombectomy for assessment of change in right ventricular function and/or pulmonary A (7) artery pressure Cardiac Trauma 32. Severe deceleration injury or chest trauma when valve injury, pericardial effusion, or cardiac injury is possible or suspected A (9) 33. Initial evaluation when there is a reasonable suspicion of valvular or structural heart disease A (9) 35. Initial evaluation when there is very low suspicion of valvular or structural heart disease I (2) 36. Reevaluation in a patient without valvular disease on a previous echocardiogram and no change in clinical status or findings on cardiac examination I (1) 37. Reevaluation of known valvular heart disease with a change in clinical status or findings on cardiac examination or to guide therapy A (9) Native Valvular Stenosis 38. Routine (<3 years) reevaluation of mild valvular stenosis without change in clinical status or findings on cardiac examination I (3) 39. Routine (≥3 years) reevaluation of mild valvular stenosis without change in clinical status or findings on cardiac examination A (7) 40. Routine (<1 year) reevaluation of moderate or severe valvular stenosis without change in clinical status or findings on cardiac examination I (3) 41. Routine (≥1 year) reevaluation of moderate or severe valvular stenosis without change in clinical status or findings on cardiac examination A (8) Native Valvular Regurgitation 42. Routine (<3 years) reevaluation of mild valvular regurgitation without change in clinical status or findings on cardiac examination I (1) 44. Routine (≥3 years) reevaluation of mild valvular regurgitation without change in clinical status or findings on cardiac examination U (4) 45. Routine (<1 year) reevaluation of moderate or severe valvular regurgitation without change in clinical status or findings on cardiac examination U (6) 46. Routine (≥1 year) reevaluation of moderate or severe valvular regurgitation without change in clinical status or findings on cardiac examination A (8) Prosthetic Valve 47. Initial postoperative evaluation of prosthetic valve for establishment of baseline A (9) 48. Routine (<3 years) reevaluation of prosthetic valve if no known or suspected valve dysfunction I (3) 49. Routine (≥3 years) reevaluation of prosthetic valve if no known or suspected valve dysfunction A (7) 50. Evaluation of prosthetic valve with suspected dysfunction or change in clinical status or findings on cardiac examination A (9) 51. Reevaluation of known prosthetic valve dysfunction when it would change management or guide therapy A (9) Infective Endocarditis (Native or Prosthetic Valves) 52. Initial evaluation of suspected infective endocarditis with positive blood cultures or a new murmur A (9) 53. Transient bacteremia with a pathogen not typically associated with infective endocarditis and/or documented nonendovascular source of infection I (3) 55. Reevaluation of infective endocarditis at high risk for progression or complication or with a change in clinical status or findings on cardiac examination A (9) 56. Routine reevaluation of known small pericardial effusion with no change in clinical status I (2) 61. Reevaluation of known pericardial effusion to guide management or therapy A (8) 62. Evaluation of the ascending aorta in the setting of a known or suspected connective tissue disease or genetic condition that predisposes to aortic aneurysm A (9) or dissection (e. Reevaluation of known ascending aortic dilation or history of aortic dissection to establish a baseline rate of expansion or when the rate of expansion is A (9) excessive 65. Reevaluation of known ascending aortic dilation or history of aortic dissection with a change in clinical status or findings on cardiac examination or when A (9) findings may alter management or therapy 66. Routine evaluation of systemic hypertension without suspected hypertensive heart disease I (3) 69. Reevaluation of known hypertensive heart disease without change in clinical status or findings on cardiac examination U (4) Heart Failure 70. Initial evaluation of known or suspected heart failure (systolic or diastolic) based on symptoms, signs, or abnormal test results A (9) 71. Reevaluation of known heart failure (systolic or diastolic) with change in clinical status or findings on cardiac examination and no clear precipitating change A (8) in medication or diet 72. Reevaluation of known heart failure (systolic or diastolic) with change in clinical status or findings on cardiac examination and a clear precipitating change U (4) in medication or diet 73. Reevaluation of known heart failure (systolic or diastolic) to guide therapy A (9) 74. Routine (<1 year) reevaluation of heart failure (systolic or diastolic) when there is no change in clinical status or findings on cardiac examination I (2) 75. Routine (≥1 year) reevaluation of heart failure (systolic or diastolic) when there is no change in clinical status or findings on cardiac examination U (6) Device Evaluation (Including Pacemaker, Implantable Cardioverter-Defibrillator, or Cardiac Resynchronization Therapy) 76. Initial evaluation or reevaluation after revascularization and/or optimal medical therapy to determine candidacy for device therapy and/or to determine A (9) optimal choice of device 77. Initial evaluation for optimization of device for cardiac resynchronization therapy after implantation U (6) 78. Known implanted pacing device with symptoms possibly caused by device complication or suboptimal pacing device settings A (8) 79.
Awareness of this heuristic and its pitfalls can help clinicians avoid this common reasoning error premarin 0.625mg on line women's health bendigo phone number. Their advantage is that buy cheap premarin 0.625mg menopause jealousy, unlike sensitivity and specificity 0.625mg premarin sale fsh 80 menopause, likelihood ratios are dimensionless numbers order 0.625mg premarin overnight delivery menstrual hormone cycle, so the need to keep track of the numerator and denominator is alleviated. Likelihood ratios give a measure of the persuasiveness of a positive and negative test result and can be used intuitively or used to calculate post-test odds. A likelihood ratio is defined as the percentage of diseased patients with a given test result divided by the percentage of nondiseased patients with that same test result. It is easy to calculate the positive and negative likelihood ratios from sensitivity and specificity. Once calculated, these numbers can be used to multiply the pretest odds to calculate the post-test odds of a diagnosis. They are multipliers, so a higher positive likelihood ratio and a lower negative likelihood ratio (which is a fraction) have stronger multiplying effects. A likelihood ratio that is close to 1 is weak because it would have very weak multiplying effect, meaning it has minimal effect on the pretest assessment. Some tests are asymmetric, meaning that their positive or negative likelihood ratio is stronger. For example, congestion on a chest x-ray film has a very strong positive likelihood ratio of 13. This reflects that the chest radiograph is highly specific but not very sensitive for heart failure. In other words, congestive findings on a chest radiograph are highly suggestive of heart failure, whereas their absence would not be reassuring about lack of heart failure. Tests that are highly specific are better for ruling in a diagnosis, and this can be remembered using the mnemonic “SpPin” (highly specific tests, if positive, are good for ruling in). On the other hand, a D- dimer for a pulmonary embolus has a very strong negative likelihood ratio of 0. This reflects that a D-dimer is highly sensitive but not very specific for pulmonary embolus. Tests that are highly sensitive are better for ruling out a diagnosis, and this can be 19 remembered using the mnemonic “SnNout” (highly sensitive tests, if negative, are good for ruling out). The likelihood ratios, however, are only as useful and precise as the sensitivity and specificity that are used to calculate them. They give an approximate quantitative estimate of the strength of new information that provides a mechanism for calibrating intuitive probability estimates. When used with odds, likelihood ratios provide a way to calculate the conditional probabilities that are used for bayesian reasoning. Proceeding through this calculation demonstrates the conceptual framework for reasoning through iterative hypothesis testing. Test-Ordering Strategies Clinical reasoning should guide not only test interpretation, but also test ordering. Tests that are ordered for good reasons are more conclusive, and tests that are ordered indiscriminately can cause clinicians to arrive at the wrong conclusions. This effort is driven by both the need to avoid excessive false-positive test results and the need to contain the costs of medical care. The goal of appropriate-use guidelines is to reduce overuse errors and maximize the value of diagnostic testing and procedures. The general principle of any test- ordering strategy is that a plausible hypothesis (a provisional diagnosis) should be formulated first, followed by testing. The appropriate-use criteria are designed to avoid testing when the results are unlikely to improve patient care or outcomes. These guideline recommendations emphasize the need to consider categories based on estimates of risk and prognosis, rather than diagnostic labels. Risk is another word for probability, and when used in this context, risk takes on a meaning of propensity, which is probability that has a modifiable tendency or disposition. It is important for clinicians to understand how risk is calculated from long-term observations from pooled cohorts of test subjects, in order to understand the strengths and limitations of these risk calculations. After calculating the risk, the challenge for clinicians is communicating risk to patients in an understandable way. Investigators have provided pictograms that can communicate risk and risk reduction to facilitate a discussion regarding long-term treatment options to diminish risk and to compare the degree of risk reduction with potential side effects and costs of treatment. Since clinicians vary in their use of qualitative terms, such as “high risk,” there is a need to provide clear and understandable quantitative estimates. Therapeutic Decisions A preventive or therapeutic decision is a structured choice. For some situations, it is a simple and easy decision, such as deciding to give a diuretic to a patient with acute congestive heart failure. In this case the stakes are not high, the preference of the patient is clear, and the decision is straightforward. An elderly patient with moderate to severe mitral regurgitation and comorbid conditions presents a difficult choice based on estimated probabilities of the natural history of the disorder, versus the surgical risks and prospects for an improved outcome with a surgical intervention. Of note, the relative benefit (or risk) of an intervention is often expressed as a relative risk or odds ratio. Risk is the probability of an event, and odds is the probability an event will occur against the probability that it will not occur. The risk ratio expresses the relative probability that an event will occur when two groups are compared. The odds ratio expresses the odds of the event in one group compared with another. Despite its widespread use, the odds ratio is less helpful than relative risk in clinical decision making. The expressions are similar when baseline event rates are low (<5%), but deviate with higher risk and larger treatment effects. The odds ratio can express associations but, unlike the risk ratio, cannot express the relative size of the treatment effect; if clinicians assume odds to be equivalent to risk, it may lead to overestimates of the treatment effect when the outcome is common. The odds ratio is often used in clinical research because of its mathematical properties and its utility for identifying associations in certain situations, but clinicians need to know its limitations for estimates of treatment effect. Clinical trials report the average risk of an outcome for patients in a treatment group and in a comparison group. There may be heterogeneity of the treatment effect, in which some patients may receive a marked benefit and others receive no benefit at all. Subgroup analysis and tests for interaction can provide hints, but usually heterogeneity of treatment effect is not readily apparent, creating a challenge for clinicians trying to personalize treatment decisions. The challenge is that subgroup analyses introduce the possibility that associations have occurred only by chance. Thus, subgroup analyses are capable of 24 producing important insights, but must be interpreted with caution. Risk Stratification A weakness of relative benefit estimates is that they do not convey information about what is achieved for patients at varying levels of risk. A small relative reduction in risk may be meaningful for a high-risk patient, whereas a large relative reduction may be inconsequential for a very-low-risk patient.
Buy discount premarin 0.625mg. PERIFIT: A Revolution for Women's Health.
Anticipating this movement when initially securing the endotracheal tube and its connections will prevent disconnection cheap premarin 0.625 mg without a prescription women's health clinic cork. In neck surgery purchase premarin 0.625mg otc women's health magazine birth control article, the neck is often rotated away from the surgeon; overrotation presents the risk of brachial plexus stretch injuries safe 0.625 mg premarin menstrual irregularities. If a radial free flap is anticipated buy generic premarin 0.625mg menstruation quiz, then positioning of the arm as well as rotation of the head should be carefully coordinated to avoid injury while still providing needed access and a secure airway. For selected cases the patient also will have had preop embolization of a tumor and its blood supply (e. Bradycardia may occur if the surgeon operates near the vagus nerve or carotid bifurcation. If this occurs, it is usually sufficient for the anesthesiologist to communicate this and the surgeon can desist for a period of time. Careful H&P must be performed to ensure that the patient’s functional status is optimized. Meticulous examination of the airway must be performed, and there should be a low threshold for an awake intubation if the airway is questionable. Straining, bucking, or coughing may provoke early postop bleeding (↑ venous and arterial pressure), disrupt delicate suture lines (e. In an opioid-naive patient, the choice of an opioid analgesic depends primarily on several factors: anticipated surgical stimulation and postop pain, duration of surgery, coexisting medical conditions. High dose opioids (fentanyl: loading dose 3–10 mcg/kg iv, sufentanil: loading dose 0. For procedures that may be highly stimulating, but associated with minimal postop discomfort (e. A variety of pharmacological approaches have been successfully employed for this purpose. When used appropriately in selected patients, absence of immediate access to the patient’s airway is not a deviation from the standard of care. The2 absence of gastric insufflation should be documented in the anesthesia record after auscultating the epigastric area. Deeper stages of anesthesia are usually required until the very end of the procedure to blunt patient’s laryngo-tracheal responses. A low-dose remifentanil infusion to blunt the tracheal responses and promote smooth extubation may be helpful. The addition of metoclopromide (10– 20 mg iv) may be beneficial for the patients who had undergone the procedures resulting in accumulation of the passively swallowed blood in the stomach (e. Bitar G, Mullis W, Jacobs W, et al: Safety and efficacy of office-based surgery with monitored anesthesia care/sedation in 4778 consecutive plastic surgery procedures. Mamiya H, Ichinohe T, Kaneko Y: Negative pressure pulmonary edema after oral and maxillofacial surgery. Niamtu J: Expanding hematoma in face-lift surgery: literature review, case presentations, and caveats. Prendiville S, Weiser S: Management of anesthesia and facility in facelift surgery. The patient is supine with cervical spine flexed and atlantoaxial joint extended (this position is best achieved with a headrest); and the teeth are protected with a mouth guard. Any bleeding normally can be controlled easily with cotton pledgets soaked in epinephrine, packing, or endoscopic cautery. Laryngoscopy often is combined with esophagoscopy, bronchoscopy, or direct nasopharyngoscopy to survey the aerodigestive tract for malignancy. If the procedure is diagnostic, the surgeon may need to visualize the airway before intubation and/or muscle relaxation. Usual preop diagnosis: Oropharyngeal, hypopharyngeal, or laryngeal tumors Description: Operative microlaryngoscopy. A variety of laryngeal lesions, including papilloma, cysts, and polyps, can be removed endoscopically. Because of their close apposition to the delicate tissues of the vocal fold, a high degree of precision may be needed to remove the growth without damaging the underlying membrane. To this end, specialized endoscopes, such as the Dedo operating laryngoscope, are deployed transorally to allow the surgeon a binocular view of the vocal fold and the target lesion. They can be suspended from a Mayo stand to free both of the surgeon’s hands for operating. Because of the precision involved in such procedures and the high degree of stimulation to the patient, general anesthesia (± jet ventilation) with muscle relaxation is required. Intubation with a small-caliber microlaryngeal or laser-safe tube (5 or 6 mm) may be required for these procedures. In cases where jet ventilation is to be performed, an endoscope suitable for this technique should be available. Intermittent apneic ventilation is also a possibility, although this involves periodic interruption of surgery, which can be cumbersome and distracting. Usual preop diagnosis: Vocal fold neoplasm; vocal fold paralysis Description: Bronchoscopy is used for visualization of the tracheobronchial tree for both diagnostic and therapeutic purposes. The patient is supine with head elevated and neck extended at the upper cervical level. The bronchoscope is directed along the right side of the tongue forward toward the midline to visualize the epiglottis. Next, the bronchoscope tip is used to lift the epiglottis and advance the bronchoscope through the vocal cords, into the trachea and bronchus (Fig. With the aid of telescopes, the bronchoscope can be directed for inspection of the carina, main bronchi, and the segmental bronchi. Rigid bronchoscopes provide a large working channel through which to introduce grasping and biopsy forceps. As such, rigid bronchoscopes may provide a more stable platform for removal or retrieval of foreign bodies, tumors, and stents than flexible fiberoptic bronchoscopes. Flexible fiberoptic bronchoscopy is more commonly performed than rigid bronchoscopy. The endoscope is usually connected to a monitor, and suction, irrigation, and biopsy channels are self-integrated. A bite block is usually placed to protect the endoscope from dental trauma and to allow easier advancement through the oropharynx into the larynx. The patient is supine with head elevated and neck extended at the upper cervical level. The esophagoscope (held in the dominant hand) is advanced through the mouth behind the arytenoids, gently using the thumb of the nondominant hand. The bevel of the scope is then used to advance through the cricopharyngeal muscle (upper esophageal sphincter) with an upward lifting movement, entering the cervical esophagus. As the scope advances, the head may have to be lowered or the neck extended and the scope directed slightly toward the left. The scope is advanced to the gastroesophageal junction with great care to ensure a visible lumen is seen at all times to avoid inadvertent perforation. Flexible fiberoptic esophagoscopy is performed in an essentially identical manner.
Following pericardiocentesis discount 0.625 mg premarin mastercard menstrual cycle 8 days apart, repeat echocardiography and in many cases continued hemodynamic monitoring should be employed to assess reaccumulation buy premarin 0.625 mg visa women's health clinic in houston. Intrapericardial catheters should be left in place for several days to allow continued drainage generic premarin 0.625mg visa womens healthcare associates boca raton. This minimizes recurrence and facilitates the delivery of intrapericardial drugs if 2 effective 0.625 mg premarin women's health tips now,48,49 appropriate. Open pericardiocentesis is occasionally preferred for the initial removal of fluid. Loculated effusions and/or effusions that are borderline in size are drained more safely in the operating room. Recurring effusions, especially those causing tamponade, can initially be drained using a closed approach because of logistical considerations. However, open pericardiocentesis with biopsy and creation of a pericardial 2 window are preferred for recurrences severe enough to cause tamponade. Percutaneous balloon pericardiotomy and pericardioscopy have been employed to drain fluid, create 50,51 pericardial windows, and perform pericardial biopsy. Balloon pericardiotomy may be particularly useful for malignant effusions and other situations where recurrence is common and a definitive approach without a surgical procedure is desirable. These methods appear safe and effective, but experience is limited and confined to centers with a special interest in pericardial disease. Analysis of Pericardial Fluid 1 Normal pericardial fluid has the features of a plasma ultrafiltrate. Although routine analysis of fluid does not have a very high yield for disease etiology, analysis is rewarding with bacterial infections and malignant effusions. Although most effusions are exudates, detection of a transudate reduces diagnostic possibilities. Sanguineous fluid is nonspecific and does not necessarily indicate active bleeding. Chylous effusions can occur after traumatic or surgical injury to the thoracic duct or obstruction by neoplasms. Pericardial fluid should routinely be stained and cultured for bacteria, including Mycobacterium tuberculosis, and fungi and as much fluid as possible submitted for detection of malignant cells. If there is a suspicion of tuberculous pericarditis, at least one of these tests should be routine because of the difficulty in diagnosing this disorder and delays in making a diagnosis by culture. New and novel approaches for the analysis of pericardial fluid have been the subject of active investigation. As discussed below, there may be a role for measurement of tumor markers as a screen for 2,52 malignant effusion. Selected cytokine and related biomarkers measured in both pericardial fluid and serum have shown promise in distinguishing various types of inflammatory effusions, but their precise 53,54 roles have not been elucidated. Pericardioscopy and Percutaneous Biopsy Pericardioscopic-guided drainage of pericardial effusions was discussed earlier. When standard noninvasive methods of evaluating the cause of pericardial effusions are unsuccessful, extended pericardioscopically guided biopsies combined with a battery of immunologic and molecular methods applied to both fluid and tissue (e. However, experience is limited and it is not known whether such an approach will in fact improve long-term outcomes. Constrictive Pericarditis Etiology Constrictive pericarditis is the end stage of an inflammatory process involving the pericardium. In the developed world the disorder is most 1,2,56,57 commonly idiopathic or due to surgical complications or radiation injury. Constriction can follow an initial insult by as little as several months and occasionally less, but typically takes years to develop. The end result is fibrosis, often calcification, and adhesions of the parietal and visceral pericardium. Scarring is usually more or less symmetric and impedes filling of all heart chambers. In a subset of patients, constriction is transient and/or reversible by antiinflammatory drugs. This is observed early after cardiac surgery and in 1,59-62 other patients with intense pericardial inflammation (discussed below). Pathophysiology 1,2 The consequence of pericardial scarring is markedly restricted filling of the heart. This results in elevated and equal filling pressures in all chambers and systemic and pulmonary veins. In early diastole the ventricles fill rapidly because of markedly elevated atrial pressures and accentuated early diastolic ventricular suction related to small end-systolic volumes. During early to mid-diastole, ventricular filling abruptly ceases when the cardiac volume reaches the limit set by the pericardium. Systemic venous congestion results in hepatic congestion, peripheral edema, ascites, anasarca, and cardiac cirrhosis. Reduced cardiac output also results from impaired filling and causes fatigue, muscle wasting, and weight loss. The myocardium is occasionally involved in inflammation and fibrosis, leading to contractile dysfunction, which predicts a 63 poor result after pericardiectomy. Failure of transmission of intrathoracic respiratory pressure changes to the cardiac chambers through the thickened pericardium is an important contributor to the pathophysiology of constrictive pericarditis (Fig. On inspiration, the drop in intrathoracic pressure is transmitted to the pulmonary veins but not 1 the left heart. High systemic venous pressure and reduced cardiac output induce retention of sodium and water by the kidneys. During inspiration the decrease in left ventricular filling results in a leftward septal shift, allowing augmented flow into the right ventricle. Clinical Presentation The usual presentation consists of signs and symptoms of right heart failure, including lower extremity edema, vague abdominal complaints, and passive hepatic congestion. With progression, hepatic congestion worsens and can progress to ascites, anasarca, and jaundice due to cardiac cirrhosis. Signs and symptoms of left heart failure, dyspnea, cough, and orthopnea may also appear. Atrial fibrillation and tricuspid regurgitation, which further exacerbate venous pressure elevation, are common at this stage. At the end stage, effects of a chronically low cardiac output are prominent, including fatigue, muscle wasting, and cachexia. Constrictive pericarditis can be mistaken for any cause of right heart failure, as well as end-stage liver disease. Physical Examination Physical findings include markedly elevated jugular venous pressure with a prominent, rapidly collapsing y descent. This, combined with a normal x descent, results in an M- or W-shaped venous pressure contour.